Advertisements
Advertisements
प्रश्न
What does one mean by exothermic reaction? Give example.
उत्तर १
Reactions in which heat is released along with the formation of products are called exothermic chemical reactions.
Example: \[\ce{C(s) + O2(g) -> CO2(g) + Heat}\]
\[\ce{2H2(g) + O2(g) -> 2H2O(l) + Heat}\]
उत्तर २
A reaction in which heat is released is called an exothermic reaction. Burning of fuel is an example of exothermic reaction. When methane is burned, it gives heat along with carbon dioxide and water.
\[\ce{CH4(g) + 2O2(g) -> CO2(g) + 2H2O(l) + Heat}\]
संबंधित प्रश्न
Taking into consideration the relationship in the first pair, complete the second pair
2H2 + O2 → 2H2O :Combination Reaction :: 2HgO → 2Hg + O2:_________
Give one example of a decomposition reaction which is carried out by applying heat.
What type of reaction is represented by the following equation?
NH4 CI → NH3 + HCI
What type of chemical reaction take place when lime-stone is heated?
What type of chemical reaction take place when electricity is passed through water?
What type of chemical reaction is represented by the following equation?
X → Y + Z
When a green iron salt is heated strongly, its colour finally changes to brown and odour of burning sulphur is given out.
(a) Name the iron salt.
(b) Name the type of reaction that takes place during the heating of iron salt.
(c) Write a chemical equation for the reaction involved.
Study the following figure and answer questions.

a) After heating Calcium carbonate, which gas is formed in a test tube?
b) When we pass this gas through limewater what change, did you observe?
c) Write down the chemical reaction showing the product formation after heating the Calcium carbonate.
A student wants to study a decomposition reaction by taking ferrous sulphate crystals. Write two precautions he must observe while performing the experiment.
Give a balanced equation for –
An electrolytic decomposition reaction involving a neutral liquid
Give a balanced equation for the following type of reaction:
A thermal decomposition reaction in which a metallic nitrate decomposes to give – a basic oxide.
What is electrolysis?
The following reaction is used for the preparation of oxygen gas in the laboratory:
\[\ce{2KClO3_{(s)} ->[Heat] 2KCl + 3O2_{(g)}}\]
Which of the following statement about the reaction is correct?
Which one of the following processes involves chemical reactions?
Assertion: Decomposition of vegetable matter into compost is an endothermic reaction.
Reason: Decomposition reaction involves breakdown of a single reactant into simpler products.
Marble statues are corroded or stained rain water. Identify the main reason.
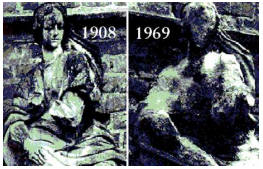
These consist of two statements – Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
Assertion: Silver bromide decomposition is used in black and white photography.
Reason: Light provides energy for this exothermic reaction.
What is observed when silver chloride is exposed to sunlight? Give the type of reaction involved.
A solution of a substance ‘X’ is used for whitewashing.
- Name the substance ‘X’ and write its formula.
- Write the reaction of the substance ‘X’ named in the above formula with water.
