Advertisements
Advertisements
प्रश्न
What does one mean by exothermic reaction? Give example.
उत्तर १
Reactions in which heat is released along with the formation of products are called exothermic chemical reactions.
Example: \[\ce{C(s) + O2(g) -> CO2(g) + Heat}\]
\[\ce{2H2(g) + O2(g) -> 2H2O(l) + Heat}\]
उत्तर २
A reaction in which heat is released is called an exothermic reaction. Burning of fuel is an example of exothermic reaction. When methane is burned, it gives heat along with carbon dioxide and water.
\[\ce{CH4(g) + 2O2(g) -> CO2(g) + 2H2O(l) + Heat}\]
संबंधित प्रश्न
A solid substance P which is very hard is used in the construction of many buildings, especially flooring. When substance P is heated strongly, it decomposes to form another solid Q and a gas R is given out. Solid Q reacts with water with the release of a lot of heat to form a substance S. When gas R is passed into a clear solution of substance S, then a white precipitate of substance T is formed. The substance T has the same chemical composition as starting substance P.
(a) What is substance P? Write its common name as well as chemical formula.
(b) What is substance Q?
(c) What is gas R?
(d) What is substance S? What is its clear solution known as?
(e) What is substance T? Name any two natural forms in which substance T occurs in nature.
Give one example of a decomposition reaction which is carried out by applying heat.
What type of chemical reaction take place when silver bromide is exposed to sunlight?
Name the product formed on strongly heating ferrous sulphate crystals. What type of chemical reaction occurs in this change?
Classify the following reaction as combination, decomposition, displacement, precipitation and neutralization. Also balance the equation.
\[\ce{Zn_{(s)} + H2SO4 -> ZnSO4_{(s)} + H2_{(g)}}\]
How will you obtain Nitrogen dioxide from lead nitrate.
Also give balanced equations for the reactions
What are thermal decomposition reactions ? Explain with an example.
Give scientific reason.
When the gas formed on heating limestone, is passed through freshly prepared lime water, the lime water turns milky.
Answer the following question.
2 g of silver chloride is taken in a china dish and the china dish is placed in sunlight for some time. What will be your observation in this case? Write the chemical reaction involved in the form of a balanced chemical equation. Identify the type of chemical reaction.
Give a balanced equation for –
A white precipitate obtained during a double decomposition reaction involving a silver salt with sodium salt.
The chemical reaction in which two or more products are formed from a single reactant is called _______ reaction.
Photolysis is a decomposition reaction caused by ______
Electrolysis is type of ______ reaction.
Which of the following is an endothermic process?
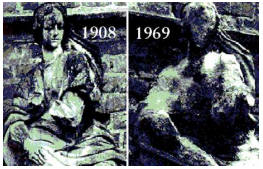
Marble statues are corroded or stained rain water. Identify the main reason.
These consist of two statements – Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
Assertion: Silver bromide decomposition is used in black and white photography.
Reason: Light provides energy for this exothermic reaction.
Write the molecular formula of calcium carbonate.
A solution of a substance ‘X’ is used for whitewashing.
- Name the substance ‘X’ and write its formula.
- Write the reaction of the substance ‘X’ named in the above formula with water.
