Advertisements
Advertisements
प्रश्न
If same mass of liquid water and a piece of ice is taken, then why is the density of ice less than that of liquid water?
उत्तर
The mass per unit volume (i.e., mass/volume) is called density. Since water expands on freezing, therefore, volume of ice for the same mass of water is more than liquid water. In other words, density of ice is lower than liquid water and hence ice floats on water.
APPEARS IN
संबंधित प्रश्न
Among NH3, H2O and HF, which would you expect to have highest magnitude of hydrogen bonding and why?
Why is it said that-
There is no alternative to water for cleaning purposes.
Sea water is a bad conductor of electricity.
Explain the picture in your own words.
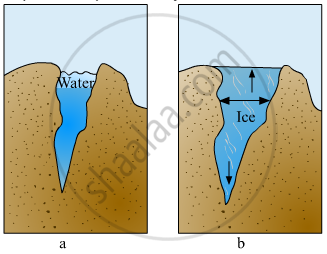
Define the following.
Boiling point
Which of the following equation depicts reducing nature of \[\ce{H2O2}\]?
Melting point, enthalpy of vapourisation and viscosity data of \[\ce{H2O}\] and \[\ce{D2O}\] is given below :
| \[\ce{H, O}\] | \[\ce{D2O}\] | |
| Melting point / K | 373.0 | 374.4 |
| Enthalpy of vapourisation at (373 K)/kJ mol–1 | 40.66 | 41.61 |
| Viscosity/centipoise | 0.8903 | 1.107 |
On the basis of this data explain in which of these liquids intermolecular forces are stronger?
Explain why \[\ce{HCl}\] is a gas and \[\ce{HF}\] is a liquid.
Which of the following ion is responsible for the temporary hardness of water?
The density of gold is 19 g/cm3. If 1.9 × 10−4 g of gold is dispersed in one litre of water to give a sol having spherical gold particles of radius 10 nm, the number of gold particles per mm3 of the sol will be ______ × 106.
