Advertisements
Advertisements
प्रश्न
In a crystal of diamond:
(i) How many carbon atoms are present per unit cell?
(ii) What type of lattice does diamond crystallize in?
(iii) How many carbon atoms surround each carbon atom?
(iv) How are they arranged?
उत्तर
i)
Four carbon atoms are present per unit cell.
(ii)
Diamond crystallizes in a network type of lattice with the free arrangement of carbon atoms.
(iii)
Four carbon atoms surround each carbon atom.
(iv)
Four carbon atoms are tetrahedrally arranged around each central carbon atom.
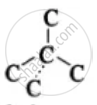
Tetrahedral arrangement
APPEARS IN
संबंधित प्रश्न
What is the formula of a compound in which the element Y forms ccp lattice and atoms of X occupy 1/3rd of tetrahedral voids?
Define the following term: Primitive unit cells
An ionic crystal lattice has limiting value of radius ratio as 0.414 to 0.732; the co-ordination number of its cation is _______.
(A) 6
(B) 4
(C) 3
(D) 12
Which of the following parameters are correct for triclinic lattice?
(A) α = β = γ = 90° and a = b = c
(B) α ≠ β ≠ γ = 90° and a ≠ b ≠ c
(C) α = γ = 90°, β ≠ 90° and a ≠ b ≠ c
(D) α ≠ β ≠ γ ≠ 90° and a ≠ b ≠ c
How will you obtain pure potassium permanganate (KMnO4) crystals from its ore, pyrolusite? Give the steps involved and the reactions.
A solid compound XY has NaCl structure. if the radius of the cation is 100 pm, the radius of the anion will be
Why ionic crystals are hard and brittle?
How many three-dimensional crystal lattices are possible?
AB crystallises in a body centred cubic lattice with edge length' a' equal to 387 pm The distance between two oppositely charged ions in the lattice is:
How many unit cells are present in a cube-shaped ideal crystal of NaCI of mass 1.00 g? [Atomic masses : Na = 23, Cl = 35.5)
