Advertisements
Advertisements
प्रश्न
In the laboratory preparation of hydrogen from zinc and dil. acid. Give a reason for the following:
The complete apparatus is air-tight.
उत्तर
The gas is highly inflammable, any leakage can cause an explosion.
APPEARS IN
संबंधित प्रश्न
Indicate which of the following statement is true and which is false:
Zinc can liberate hydrogen from water, acid and alkali solution.
Give reason for the following:
Nitric acid not used for the preparation of hydrogen gas?
Name the following:
A metal which liberates hydrogen only when steam is passed over red hot metal.
Name the chemicals required to prepare hydrogen gas in the laboratory.
Draw a neat and well-labelled diagram for the laboratory preparation of hydrogen.
How would you show that hydrogen is a non-supporter of combustion?
FILL IN THE BLANK
Sodium liberates hydrogen when treated with cold .............
Give a test to identify hydrogen ?
Name the impurities present in hydrogen prepared in the laboratory.
How would you show that hydrogen is lighter than air?
Starting from zinc how would you obtain hydrogen using Steam.
[Give balanced equation & name the product formed in the case other than hydrogen].
Name a metal which will not react with the reactants above to give hydrogen.
In the laboratory preparation of hydrogen from zinc & dilute hydrocholoric acid – state a reason for the addition of traces of copper [II] sulphate to the reaction medium.
Draw neat labelled diagrams for two different experiments to prove that – hydrogen is lighter than air.
Give a balanced equation for the following conversions sodium aluminate from aluminium.
In the laboratory preparation of hydrogen from zinc and dil. acid. Give a reason for the following:
The lower end of the thistle funnel should dip below the level of the acid in the flask.
Name the following.
A gaseous reducing agent which is basic in nature.
The diagram represent the preparation and collection of hydrogen by a standard laboratory method.
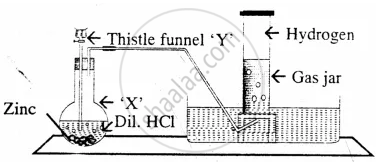
Name a solution which absorbs the impurity – `"H"_2"S"`
The diagram represent the preparation and collection of hydrogen by a standard laboratory method.

Name a gas other than hydrogen collected by the same method.
