Advertisements
Advertisements
प्रश्न
Match the starting materials given in Column I with the products formed by these (Column II) in the reaction with HI.
| Column I | Column II | ||
| (i) | CH3—O—CH3 | (a) | 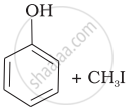 |
| (ii) | \[\begin{array}{cc} \ce{CH3}\phantom{..................}\\ \backslash\phantom{.............}\\ \ce{CH-O-CH3}\\ /\phantom{..............}\\ \ce{CH3}\phantom{..................} \end{array}\] |
(b) | \[\begin{array}{cc} \ce{CH3}\phantom{....}\\ |\phantom{.......}\\ \ce{CH3-C-I + CH3OH}\\ |\phantom{.......}\\ \ce{CH3}\phantom{....} \end{array}\] |
| (iii) | \[\begin{array}{cc} \ce{CH3}\phantom{.}\\ |\phantom{....}\\ \ce{H3C-C-O-CH3}\\ |\phantom{....}\\ \ce{CH3}\phantom{..} \end{array}\] |
(c) | 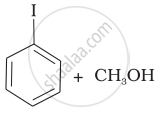 |
| (iv) | 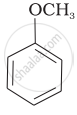 |
(d) | CH3—OH + CH3—I |
| (e) | \[\begin{array}{cc} \ce{CH3}\phantom{.....................}\\ \backslash\phantom{.................}\\ \ce{CH-OH + CH3I}\\ /\phantom{.................}\\ \ce{CH3}\phantom{.....................} \end{array}\] |
||
| (f) | \[\begin{array}{cc} \ce{CH3}\phantom{.....................}\\ \backslash\phantom{.................}\\ \ce{CH-I + CH3OH}\\ /\phantom{.................}\\ \ce{CH3}\phantom{.....................} \end{array}\] |
||
| (g) | \[\begin{array}{cc} \ce{CH3}\phantom{....}\\ |\phantom{.......}\\ \ce{CH3-C-OH + CH3I}\\ |\phantom{.......}\\ \ce{CH3}\phantom{....} \end{array}\] |
उत्तर
| Column I | Column II | ||
| (i) | CH3—O—CH3 | (d) | CH3—OH + CH3—I |
| (ii) | \[\begin{array}{cc} \ce{CH3}\phantom{..................}\\ \backslash\phantom{.............}\\ \ce{CH-O-CH3}\\ /\phantom{..............}\\ \ce{CH3}\phantom{..................} \end{array}\] |
(e) | \[\begin{array}{cc} \ce{CH3}\phantom{.....................}\\ \backslash\phantom{.................}\\ \ce{CH-OH + CH3I}\\ /\phantom{.................}\\ \ce{CH3}\phantom{.....................} \end{array}\] |
| (iii) | \[\begin{array}{cc} \ce{CH3}\phantom{.}\\ |\phantom{....}\\ \ce{H3C-C-O-CH3}\\ |\phantom{....}\\ \ce{CH3}\phantom{..} \end{array}\] |
(b) | \[\begin{array}{cc} \ce{CH3}\phantom{....}\\ |\phantom{.......}\\ \ce{CH3-C-I + CH3OH}\\ |\phantom{.......}\\ \ce{CH3}\phantom{....} \end{array}\] |
| (iv) |  |
(a) |  |
Explanation:
(i) \[\ce{CH3 - O - CH3}\] is a symmetrical ether so the products are \[\ce{CH3I}\] and \[\ce{CH2OH}\].
(ii) In \[\ce{(CH3)2CH – O – CH3}\] unsymmetrical ether, one alkyl group is primary while another is secondary. So, it follows SN2 mechanism.
(iii) In this case, one of the alkyl group is tertiary and the other is primary. It follows SN1 mechanism and halide ion attacks the tertiary alkyl group and the products are \[\ce{(CH3)3 C-I}\] and \[\ce{CH3OH}\].
(iv) Here, the unsymmetrical ether is alkyl aryl ether. In this ether \[\ce{O - CH3}\] bond is weaker than \[\ce{O - C6H5}\] bond which has partial double bond character due to resonance. So, the halide ion attacks on alkyl group and the products are \[\ce{C6H5 - OH}\] and \[\ce{CH3I}\].
APPEARS IN
संबंधित प्रश्न
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{................}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3\phantom{.}}\phantom{..}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
Propanoic acid to ethylamine.
Write structural formulae for Pentane-1,4-diol
Which of the following is most acidic?
The major product formed by the reaction:
\[\begin{array}{cc}
\ce{CH3CH-CH2Br ->[CH3O^-][CH3OH] is}\\
|\phantom{................}\\
\ce{CH3}\phantom{.............}
\end{array}\]
\[\ce{HC ≡ CH ->[HgSO4][H2SO4] ->[CH3MgBr][H2O] ->[PBr3]}\]
Explain why Lewis acid is not required in bromination of phenol?
How can phenol be converted to aspirin?
How are the following conversions carried out?
Methyl magnesium bromide→2-Methylpropan-2-ol.
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{................}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{..}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
