Advertisements
Advertisements
प्रश्न
How can phenol be converted to aspirin?
उत्तर
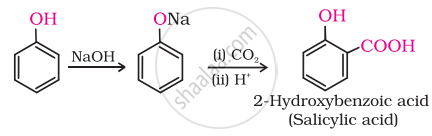
2-hydroxybenzoic acid on treatment with acetic anhydride will give asprin.
APPEARS IN
संबंधित प्रश्न
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{HO - CH2 - CH - CH2 - OH}\\
|\phantom{..}\\
\ce{OH}
\end{array}\]
Give IUPAC name of the following ether:
CH3CH2CH2OCH3
Write structural formulae for Methyl vinyl ether.
Write IUPAC names of the following
The compound HOCH2 – CH2OH is __________.
\[\ce{HC ≡ CH ->[HgSO4][H2SO4] ->[CH3MgBr][H2O] ->[PBr3]}\]
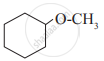
Match the structures of the compounds given in Column I with the name of the compounds given in Column II.
| Column I | Column II | |
| (i) | 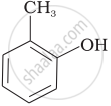 |
(a) Hydroquinone |
| (ii) | 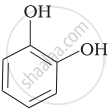 |
(b) Phenetole |
| (iii) | 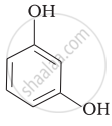 |
(c) Catechol |
| (iv) | 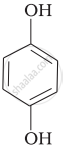 |
(d) o-Cresol |
| (v) | 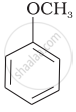 |
(e) guinone |
| (vi) | 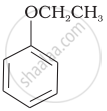 |
(f) Resorcinol |
| (g) Anisole |
Assertion: p-nitrophenol is more acidic than phenol.
Reason: Nitro group helps in the stabilisation of the phenoxide ion by dispersal of negative charge due to resonance.
Assertion: Phenol forms 2, 4, 6 – tribromophenol on treatment with \[\ce{Br2}\] in carbon disulphide at 273 K.
Reason: Bromine polarises in carbon disulphide.
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{................}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{...}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
