Advertisements
Advertisements
प्रश्न
Mention the conditions required to maximise the yield of ammonia.
उत्तर १
Ammonia is prepared using the Haber’s process. The yield of ammonia can be maximized under the following conditions:
(i) High pressure (∼ 200 atm)
(ii) A temperature of ∼700 K
(iii) Use of a catalyst such as iron oxide mixed with small amounts of K2O and Al2O3
उत्तर २
Ammonia is prepared by Haber’s process as given below:
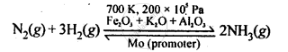
`triangle_rH^@ = -92.4 kJ mol^(-1)`
According to Le Chatelier's principle to maximise the yield of ammonia high P and T=~700 K should be used. The catalyst increases the rate of reaction and Mo promoter increase the efficiency of Fe catalyst.
APPEARS IN
संबंधित प्रश्न
Describe the laboratory method of preparation of ammonia
How does ammonia react with a solution of Cu2+?
What happens when (NH4)2Cr2O7 is heated? Write the equations.
What is the action of Excess of chlorine on ammonia?
What is the action of Na Metal on ammonia?
In laboratory ammonia is prepared by heating:
Ammonia has a higher boiling point and is less volatile because of ____________.
On heating ammonium dichromate and barium azide separately we get ______.
In Haber’s process for the manufacture of NH3:
Ammonia on reaction with hypochlorite anion can form:
Liquid ammonia bottles are opened after cooling them in ice for some time. It is because liquid NH3 ____________.
A brown ring is formed in the ring test for \[\ce{NO3^{-}}\] ion. It is due to the formation of ______.
In the preparation of HNO3, we get NO gas by catalytic oxidation of ammonia. The moles of NO produced by the oxidation of two moles of NH3 will be ______.
\[\ce{PCl5}\] reacts with finely divided silver on heating and a white silver salt is obtained, which dissolves on adding excess aqueous \[\ce{NH3}\] solution. Write the reactions involved to explain what happens.
What happens when reactions:
Benzylchloride is treated with ammonia followed by the reaction with Chloromethane.
