Advertisements
Advertisements
प्रश्न
Metal oxides generally react with acids, but few oxides of metal also react with bases. Such metallic oxides are:
- MgO
- ZnO
- Al2O3
- Cao
विकल्प
I and II
II and III
III and IV
I and IV
उत्तर
II and III
Explanation:
Some metal oxides, such as aluminium oxide, zinc oxide shows both acidic as well as basic behaviour. Such metal oxides that react with both acids and bases to produce salt and water are known as amphoteric oxides.
Aluminium oxide reacts with base as follows:
\[\ce{Al2O3 + 2NaOH -> \underset{(Sodium aluminate)}{2NaAlO2} + H2O}\]
ZnO is an oxide that can react with base, as shown by the equation given below:
\[\ce{ZnO + 2NaoH -> Na2ZnO2 + H2O}\]
संबंधित प्रश्न
Which of the following oxide(s) of iron would be obtained on the prolonged reaction of iron with steam?
Silver articles become black on prolonged exposure to air. This is due to the formation of
Stainless steel is very useful material for our life. In stainless steel, iron is mixed with
When a metal X is treated with cold water, it gives a basic salt Y with molecular formula XOH (Molecular mass = 40) and liberates a gas Z which easily catches fire. Identify X, Y and Z and also write the reaction involved.
Metals generally react with dilute acids to produce hydrogen gas. Which one of the following metals does not react with dilute hydrochloric acid?
Zinc sulphate forms a colourless solution in water. Will you observe any colour on adding copper turning in it?
Answer the questions based on the figure below:
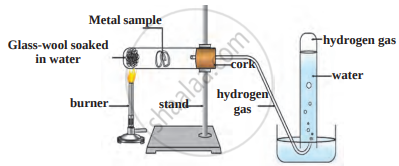
- Which reaction is shown in the figure?
- Which gas is evolved in the reaction?
- Give an example of reactants that rapidly show this reaction. Give equations.
- Give an example of reactants that do not react rapidly.
- In what condition will reactants of (c) part react? Give equation.
Select the appropriate state symbols of the products given as X and Y in the following chemical equation by choosing the correct option from table given below:
\[\ce{Zn_{(s)} + H_2SO_{4(l)} ->ZnSO_{4(X)} + H_{2(Y)}}\]
Write a chemical equation showing the ionic products formed on dissolving potassium hydroxide in water.
On adding dilute sulphuric acid to a test tube containing a metal ‘X’, a colourless gas is produced when a burning match stick is brought near it. Which of the following correctly represents the metal ‘X’?
