Advertisements
Advertisements
प्रश्न
Name one chemical compound which can be used to distinguish between ethanol and ethanoic acid.
उत्तर
Sodium hydrogencarbonate is the compound which can be used to distinguish between ethanol and ethanoic acid. It gives effervescence of carbon dioxide, with ethanoic acid.
APPEARS IN
संबंधित प्रश्न
Complete the following chemical equations : C2H5OH + Na →
Fill in the blanks from the choices given within brackets:
The basicity of acetic acid is-------- (3, 1, 4).
Draw the structure of butanoic acid.
Complete the following equation:
`CH_3 COOH + CH_2 H_5 OH` 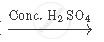
What happens when propanoic acid is warmed with methanol in the presence of a few drops of concentrated sulphuric acid? Write equation of the reaction involved.
An organic compound X of molecular formula C2H4O2 gives brisk effervescence with sodium hydrogen carbonate. Give the name and formula of X.
What type of compound is formed by the reaction between acetic acid and an alcohol?
Identify the term or substance based on the descriptions given below:
Ice like crystals formed on cooling an organic acid sufficiently.
The reaction between acetic acid and sodium carbonate is shown in the following figure.
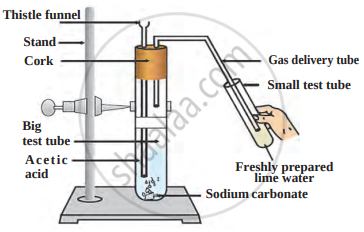
Answer the questions with the help of a diagram.
- Which gas does come out as effervescence in the big test tube?
- What is the colour change in the lime water present in the small test tube?
- Write the related reaction.
Give the balanced chemical equation of the following reaction:
Neutralization of NaOH with ethanoic acid.
