Advertisements
Advertisements
प्रश्न
Name two metals which cannot displace hydrogen from dilute acids.
उत्तर
Copper and silver are two metals which cannot displace hydrogen from dilute acids.
APPEARS IN
संबंधित प्रश्न
Complete and balance the following equation:
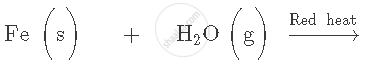
Fill in the following blank with suitable word:
Calcium is a ................. reactive metal than sodium.
Explain why, metals usually do not liberate hydrogen gas with dilute nitric acid.
How do non-metals react with hydrogen? Explain with an example.
What happens when calcium reacts with chlorine? Write an equation for the reaction which takes place
Hydrogen is not a metal but still it has been assigned a place in the reactivity series of metals. Why?
Which non-metal has been placed in the reactivity series of metals?
An element X forms two oxides XO and XO2. The oxide XO is neutral but XO2 is acidic in nature. The element X is most likely to be:
(a) sulphur
(b) carbon
(c) calcium
(d) hydrogen
An element is soft and can be cut with a knife. If is very reactive and cannot be kept open in the air. It reacts vigorously with water. The element is most likely to be:
(a) Mg
(b) S
(c) P
(d) Na
Write the name and formula of a molecule made up of three atoms of oxygen.
