Advertisements
Advertisements
प्रश्न
Quicklime is not used to dry \[\ce{HCl}\] gas because _____.
विकल्प
\[\ce{CaO}\] is alkaline.
\[\ce{CaO}\] is acidic.
\[\ce{CaO}\] is neutral.
उत्तर
Quicklime is not used to dry \[\ce{HCl}\] gas because `bbunderline(CaO "is alkaline")`.
APPEARS IN
संबंधित प्रश्न
State the inference drawn from the following observations:
Salt S is prepared by reacting dilute sulphuric acid with copper oxide. Identify S.
Give reason for the following:
When concentrated sulphuric acid is exposed to air, its volume increases and it becomes slightly dilute.
How are the following conversion brought about? Give equation and condition:
Dilute sulphuric acid to hydrogen.
How are the following conversion brought about? Give equation and condition:
Sucrose to sugar charcoal.
Describe the reaction that show
Dilute sulphuric acid behaves as dibasic acid.
Give main difference between drying agent and dehydrating agent.
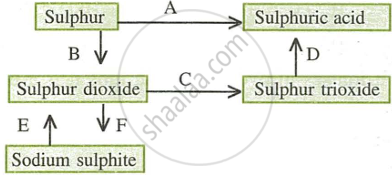
- Name the catalyst which helps in the conversion of sulphur dioxide to sulphur trioxide in step C.
- In the contact process for the manufacture of sulphuric acid, sulphur trioxide is not converted to sulphuric acid by reacting it with water. Instead a two-step procedure is used. Write the equations for the two steps involved in D.
- What type of substance will liberate sulphur dioxide from sodium sulphite in step E?
- Write the equation for the reaction by which sulphur dioxide is converted to sodium sulphite in step F.
What would you observe in the following case:
Sugar crystals are added to a hard glass test tube containing concentrated sulphuric acid.
Write balanced chemical equation to show :
The behavior of H2SO4 as an acid when it reacts with magnesium.
Which property of sulphuric acid accounts for its use as a dehydrating agent ?
