Advertisements
Advertisements
प्रश्न
Suggest a simple chemical test to distinguish propane and propene.
उत्तर
Propene decolourises Br2/H2O it forms dibromo compound but propane does not react with Br2/ H2O.
APPEARS IN
संबंधित प्रश्न
Predict the possible product of the following reaction.
sulphonation of chlorobenzene
Identify giving reason whether the following compound is aromatic or not.

Identify giving reason whether the following compound is aromatic or not.
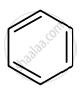
Identify giving reason whether the following compound is aromatic or not.
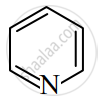
Give IUPAC name for the following compound.
\[\ce{CH ≡ C – C ≡ C – C ≡ CH}\]
Identify the compound A, B, C and D in the following series of reactions.

Sodium benzoate on decarboxylation gives ____________.
Dow's process is used for the synthesis of an aromatic compound (X). Identify X.
Which of the following molecules has shortest C - C bond length?
Read the following reaction and answer the questions given below.
\[\begin{array}{cc}
\phantom{.............................}\ce{CH3}\\
\phantom{...........................}|\\
\ce{CH3 - C = CH2 + HBr ->[benzoyl][peroxide] CH3 - CH - CH2Br}\\
|\phantom{....................................}\\
\ce{CH3}\phantom{.................................}\\
\end{array}\]
- Write IUPAC name of the product.
- State the rule that governs formation of this product.
