Advertisements
Advertisements
प्रश्न
The specific latent heat of vaporisation of steam is 2260 J/g. Comment on this.
उत्तर
The heat required to convert 1 g of water at 100°C to 1 g steam at 100°C is 2260 J.
APPEARS IN
संबंधित प्रश्न
Write the approximate value of specific latent heat of ice.
1 g ice of 0℃ melts to form 1 g water at 0℃. State whether the latent heat is absorbed or given out by ice.
Explain the following:
The heat supplied to a substance during it change of state, does not cause any rise in its temperature.
The S.I. unit of specific latent heat is ______.
During transformation of liquid phase to solid phase, the latent heat is ______.
What is meant by latent heat? How will the state of matter transform if latent heat is given off?
Answer the following:
Explain the role of latent heat in the change of state of a substance.
Liquid ammonia is used in ice factory for making ice from water. If water at 20°C is to be converted into 2 kg ice at 0°C, how many grams of ammonia are to be evaporated? (Given: The latent heat of vaporization of ammonia = 341 cal/g)
Explain the following temperature vs time graph.
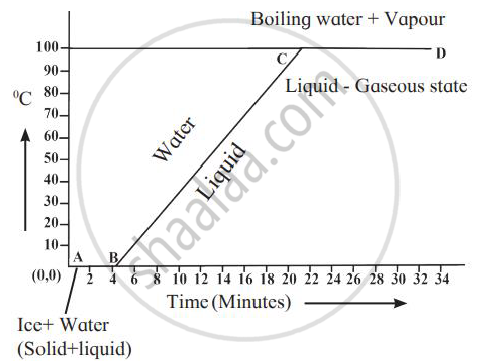
Explain the following temperature Vs. time graph:
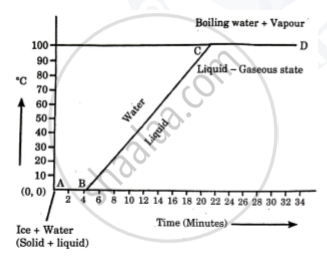
What do you mean by the statement?
'The specific latent heat capacity of fusion of ice is 336 J per g'?
What is the name given to the energy absorbed during a phase change?
Explain, why no tracks are left on the ice during ice skating?
Find the odd one out and give its explanation.
How fog is formed?
Write scientific reason.
The bottom of some steel utensils used for cooking is copper.
Define specific latent heat capacity
Who introduced the term latent heat?
600 g of copper at 50°C is mixed with lOOOg water at 20°C. Find the final temperature of the mixture. The specific heat capacity of copper is 0.4 Jg-1°C-1 and that of water is 4.2 Jg-1°C-1
