Advertisements
Advertisements
Question
The specific latent heat of vaporisation of steam is 2260 J/g. Comment on this.
Solution
The heat required to convert 1 g of water at 100°C to 1 g steam at 100°C is 2260 J.
APPEARS IN
RELATED QUESTIONS
What is the energy absorbed during the phase change called?
During transformation of liquid phase to solid phase, the latent heat is ______.
A thermally insulated pot has 150 g ice at temperature 0°C. How much steam of 100°C has to be mixed to it, so that water of temperature 50°C will be obtained? (Given : latent heat of melting of ice = 80 cal/g, latent heat of vaporization of water = 540 cal/g, specific heat of water = 1 cal/g °C)
Explain the following temperature vs time graph.
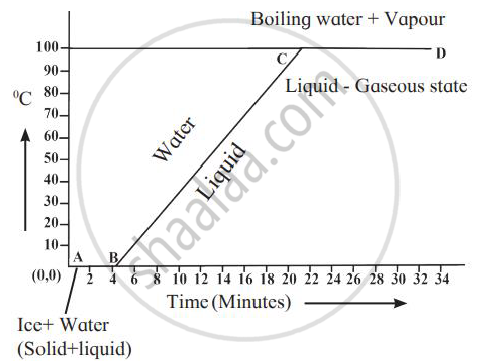
Explain the following temperature Vs. time graph:
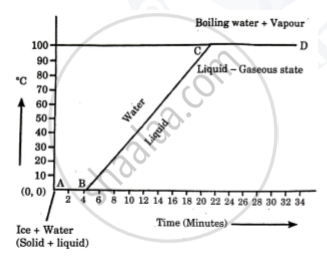
Define the following terms:
(i) Latent heat,
(ii) Latent heat of fusion of ice.
A substance changes from its solid state to the liquid state when heat is supplied to it. What name is given to heat absorbed by the substance.
Why water get cooled in a ‘Surahi’ in hot season?
Why does evaporation causes cooling and why is water used in hot water bottles?
State two advantages of the high specific latent heat capacity of steam, which is about 226 × 104 J/kg?
If pressure increases, the melting point of a substance ______.
Specific latent heat of vaporisation : J/kg : : specific heat : _______
Write the name.
The phase in which solid substances are converted into liquid.
Write scientific reason.
Even if boiling water is constantly heated, its temperature does not rise.
Write scientific reason.
Use a pressure cooker to cook food in cold air.
Write scientific reason.
The bottom of some steel utensils used for cooking is copper.
