Advertisements
Advertisements
Question
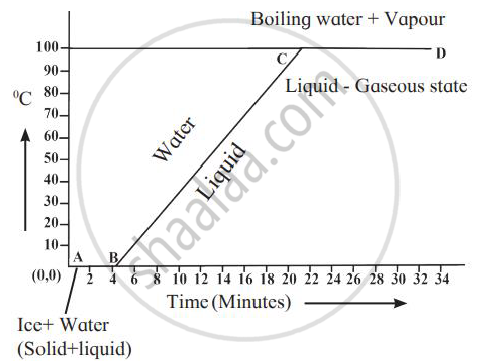
Explain the following temperature vs time graph.
Solution
- During the heating of ice, the change in temperature with time is shown in the graph.
- Seg AB - Seg AB represents the conversion of ice into the water at a constant temperature. During the melting of ice at 0°C, ice absorbs heat energy and this continues till all the ice converts into water.
- Seg BC - Once all ice is transformed into water, the temperature of water starts rising it increases up to 100°C. Seg BC represents a rise in the temperature of water from 0°C to 100°C.
- Seg CD - Even though the heat energy is supplied to the water after 100°C its temperature does not rise. The heat energy absorbed by water is used to break the bonds between molecules of the liquid to convert it into a gaseous state.
APPEARS IN
RELATED QUESTIONS
State two characteristics of a good thermion emitter.
Which has more heat: 1 g ice at 0℃ or 1g water 0℃? Give reason.
The specific latent heat of fusion of water is ______.
Explain the following temperature vs time graph.

Name two factors on which the heat absorbed or given out by a body depends.
Why does weather become pleasant when it starts freezing in cold countries?
Explain the statement; “The specific latent heat of vaporization of wafer is 2260 × 103 J/kg”.
Why do we feel much comfortable when we sit under a moving fan especially when our body is sweating?
Why does evaporation causes cooling and why is water used in hot water bottles?
State the main precautions to be taken in finding the latent heat of steam.
Calculate the total amount of heat required to convert 100g ice at 0°C to steam at 100°C.
(Specific latent heat of fusion of ice = 336 J/g, specific latent heat of vaporization of steam = 2260 J/g, specific heat capacity of water = 4.2 J/g°C).
Match the columns.
| Column A | Column B |
| 1) Absolute humidity | a) J or cal |
| 2) Latent heat | b) J/kg °C |
| 3) Specific heat capacity | c) kJ/kg |
| 4) Heat | d) no unit |
| e) kg/m3 |
During reheating, ice is converted to water at a temperature of 0 °C.
Write scientific reason.
Use a pressure cooker to cook food in cold air.
Give some practical applications of specific latent heat of ice.
Specific latent heat of a substance ______.
Calculate the total amount of heat energy required to melt 200 g of ice at 0°C to water at 100°C. (Specific latent heat of ice = 336 Jg-1, specific heat capacity of water = 4.2 Jg-1 °C-1)
