Advertisements
Advertisements
Question
Why does evaporation causes cooling and why is water used in hot water bottles?
Solution
The specific latent heat of vaporization of water is 2260 J/g = 538 calories/g. Thus when water evaporates it absorbs large amount of latent heat and thus cooling is caused. The specific heat capacity of water is 4.2 J/kg°C = 1 cal/g°C which is quite high and this is the reason for using wafer in hot water bottles.
APPEARS IN
RELATED QUESTIONS
State two characteristics of a good thermion emitter.
State two factors upon which the rate of emission of thermions depends.
- Which requires more heat: 1 g ice at 0℃ or 1 g water at 0℃ to raise its temperature to 10℃?
- Explain your answer in part (a).
Explain the following:
The surrounding become pleasantly warm when water in a lake starts freezing in cold countries.
The specific latent heat of fusion of water is ______.
A refrigerator converts 100g of water at 20℃ to ice at – 10℃ in 73.5 min. Calculate the average rate of heat extraction in watt. The specific heat capacity of water is 4.2 J kg-1 K-1, specific latent heat of ice is 336 J g-1 and the specific heat capacity of ice is 2.1 J kg-1 K-1.
During transformation of liquid phase to solid phase, the latent heat is ______.
Explain the following temperature vs time graph.
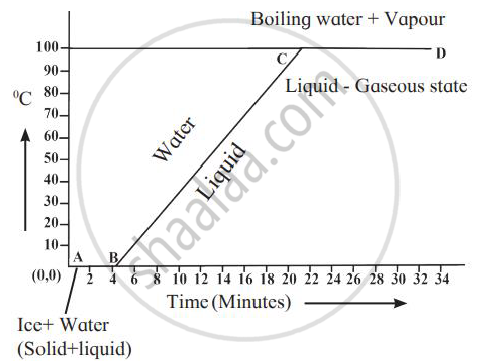
Define the following terms:
(i) Latent heat,
(ii) Latent heat of fusion of ice.
What is the name given to the energy absorbed during a phase change?
Why water get cooled in a ‘Surahi’ in hot season?
Define the term ‘specific latent heat of fusion’ of a substance.
Explain the statement; “The specific latent heat of vaporization of wafer is 2260 × 103 J/kg”.
Steam at 100°C is passed over 1000 g of ice at 0°C. After some time, 600 g of ice at 0°C is left and 450 g of water at 0°C is formed. Calculate the specific latent heat of vaporization of steam (Given: specific heat capacity of water = 4200 J/kg°C, specific latent heat of fusion of ice = 336,000 J/kg.)
When ice is converted into water : constant temperature : : before the water evaporates : _______
Calculate the amount of heat required to convert 200g of ice at 0°C into the water at 0°C Specific latent heat of fusion of ice = 336 Jg-1
Specific latent heat of a substance ______.
