Topics
Gravitation
- Concept of Gravitation
- Force
- Motion and Rest
- Centripetal Acceleration and Centripetal Force
- Kepler’s Laws
- Newton’s Universal Law of Gravitation
- Uniform Circular Motion (UCM)
- Earth’s Gravitational force
- Acceleration Due to Gravity (Earth’s Gravitational Acceleration)
- Concept of Mass and Weight
- Gravitational Waves
- Free Fall
- Gravitational Potential Energy
- Weightlessness in Space
Periodic Classification of Elements
- History of Periodic Table: Early Attempts at the Classification of Elements
- Dobereiner’s Triads
- Newland's Law of Octaves
- Mendeleev’s Periodic Table
- Merits and Demerits of Mendeleev’s Periodic Table
- Modern Periodic Law
- The Modern Periodic Table
- Structure of the Modern Periodic Table
- Modern Periodic Table and Electronic Configuration of Elements
- Groups and Electronic Configuration
- Periods and Electronic Configuration
- Periodic Properties
- Valency
- Atomic Radius Or Atomic Size
- Metallic and Non-metallic Characters
- Group VIIA Or Group 17 (The Halogens)
Chemical Reactions and Equations
- Chemical Reaction
- Chemical Equation
- Balancing Chemical Equation
- Types of Chemical Change or Chemical Reaction
- Direct Combination (or Synthesis) Reaction
- Decomposition Reactions
- Single Displacement Reactions
- Double Displacement Reaction
- Energy Change in Chemical Reactions
- Rate of Chemical Reaction
- Factors Affecting the Rate of a Chemical Reaction
- Oxidation, Reduction and Redox Reactions
- Corrosion of Metals
- Rancidity of Food and Its Prevention
Effects of Electric Current
- Electric Circuit
- Ohm's Law (V = IR)
- Heating Effect of Electric Current
- Magnetic Effect of Electric Current
- Right-hand Thumb Rule
- Magnetic Field Due to Current in a Loop (Or Circular Coil)
- Magnetic Field Due to a Current Carving Cylindrical Coil (or Solenoid)
- Force on a Current Carrying Conductor in a Magnetic Field
- Fleming’s Left Hand Rule
- Electric Motor
- Electromagnetic Induction
- Galvanometer
- Fleming’s Right Hand Rule
- Types of Current
- Electric Generator
Heat
Refraction of Light
Lenses
- Concept of Lenses
- Spherical Lens
- Convex Lens
- Images Formed by Convex Lenses
- Concave Lens
- Images Formed by Concave Lenses
- Sign Convention
- Lens Formula
- Magnification Due to Spherical Lenses
- Power of a Lens
- Combination of Lenses
- Human Eye
- Working of the Human Eye
- Eye Defect and Its Correction: Myopia Or Near-sightedness
- Eye Defect and its Correction: Hypermetropia or Far-sightedness
- Eye Defect and Its Correction: Presbyopia
- Persistence of Vision
Metallurgy
- Types of Element: Metals
- Physical Properties of Metals
- Chemical Properties of Metal
- Reactions of Metal
- Reactivity Series of Metals
- Types of Element: Non-metal
- Physical Properties of Non-metal
- Chemical Properties of Non-metal
- Ionic Compounds
- Metallurgy
- Basic Principles of Metallurgy
- Extraction of Reactive Metals
- Extraction of Aluminium
- Extraction of Moderately Reactive Metals
- Extraction of Less Reactive Metals
- Refining of Metals
- Corrosion of Metals
- Prevention of Corrosion
Carbon Compounds
- Carbon Compounds in Everyday Life
- Bonds in Carbon Compounds
- Carbon: a Versatile Element
- Properties of Carbon
- Hydrocarbons
- Structural Variations of Carbon Chains in Hydrocarbons
- Functional Groups in Carbon Compounds
- Homologous Series of Carbon Compound
- Nomenclature of Organic Compounds
- The IUPAC System of Nomenclature
- Chemical Properties of Carbon Compounds
- Ethanol
- Ethanoic Acid
- Macromolecules and Polymers
Space Missions
- Concept of Space Missions
- Artificial Satellites
- Types of Satellite
- Orbits of Artificial Satellites
- Space launch technology
- Space Missions Away from Earth
- India’s Space Programmes: Chandrayaan – 1
- India’s Space Programmes: Chandrayaan – 2
- India’s Space Programmes: Chandrayaan – 3
- India’s Space Programmes: Mangalyaan (Mars vehicle)
- India’s Space Programmes: Missions to Other Planets
- India and Space Technology
- Space Debris and Its Management
School of Elements
The Magic of Chemical Reactions
The Acid Base Chemistry
- Properties of Acids
- Strength of Acidic or Basic Solutions
- Strength of Acidic or Basic Solutions
- Acids, Bases and Their Reactivity
- Acid or a Base in a Water Solution
- Preparation and Uses of Baking Soda
- Preparation and Uses of Bleaching Powder
- Preparation and Uses of Washing Soda
- Preparation and Uses of Plaster of Paris
- Chemicals from Common Salt - Soap as a Salt
The Electric Spark
All about Electromagnetism
- Magnetic Force
- The Bar Magnet
- Right-hand Thumb Rule
- Magnetic Field Due to Current in a Loop (Or Circular Coil)
- Magnetic Field Due to a Current Carving Cylindrical Coil (or Solenoid)
- Force on a Current Carrying Conductor in a Magnetic Field
- Electric Motor
- Electromagnetic Induction
- Alternating Current (A.C.) Generator
- Direct Current Motor
- Household Electrical Circuits
Wonders of Light 1
- Spherical Mirrors
- Concave Mirror
- Concave Mirror
- Sign Convention
- Linear Magnification (M) Due to Spherical Mirrors
- Images Formed by Sperical Lenses
- Convex Lens
- Sign Convention
- Magnification Due to Spherical Lenses
- Power of a Lens
- Human Eye
- Eye Defect and Its Correction: Myopia Or Near-sightedness
- Spherical Mirrors
Wonders of Light 2
Striving for better Environment 1
- Pollution and Its Types
- Air Pollution and Its Causes
- Effects of Air Pollution
- Water Pollution and Its Causes
- Effects of Water Pollution
- Soil Pollution and its Causes
- Effects of Soil Pollution
- Noise Pollution
- Radioactive Pollution and Effects
- Abatement of Pollution
- Sustainable Use of Resources
- Introduction
- Types of Latent Heat
- Experiment
- Latent Heat of Vaporization
Introduction
Latent heat is the heat energy absorbed or released during a phase change of a substance without a change in temperature. It occurs during transitions such as:
- Solid to liquid (melting) and liquid to solid (freezing): Heat of Fusion
- Liquid to gas (vaporization) and gas to liquid (condensation): Heat of Vaporization
Latent heat is related to the enthalpy of a substance and is responsible for overcoming the attractive forces between molecules during a phase transition.
- When a solid melts into a liquid, it absorbs energy to allow molecules to move freely.
- When a gas condenses into a liquid, it releases energy, bringing molecules closer together.
- Despite absorbing or releasing heat, the temperature remains constant during the phase change.
History of Latent Heat: The concept was introduced by Joseph Black (1750–1762) while studying distillation. James Prescott Joule later described latent heat as a form of potential energy, dependent on molecular structure and bonding.
Types of Latent Heat
1. Latent Heat of Fusion (Melting and Freezing)
The heat energy required to convert a solid into a liquid at a constant temperature. Example: Ice melts into water at 0°C, absorbing heat without a temperature rise.
2. Latent Heat of Vaporization (Boiling and Condensation)
The heat energy required to convert a liquid into gas at a constant temperature. Example: Water boils at 100°C, absorbing heat until all of it turns into steam.
Experiment
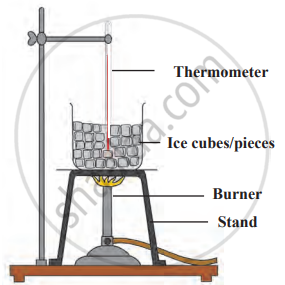
1. Aim: To study the latent heat of fusion and vapourisation by observing phase changes in water.
2. Requirements: ice cubes, beaker, thermometer, stand, burner, and stirring rod.
3. Procedure
- Place ice cubes in a beaker and insert a thermometer into the ice.
- Start heating the beaker on a burner and record the temperature every minute.
- Observe the ice melting into water and stir to maintain uniform heating.
- Continue heating the water until it reaches 100°C and begins to boil.
- Observe the temperature remaining constant until all water converts into steam.
- Plot a temperature vs. time graph to analyse phase changes.
Latent heat

Temperature vs. Time Graph
Temperature vs. Time Graph
- Line AB: Ice melts at 0°C; temperature remains constant.
- Line BC: Water heats from 0°C to 100°C; temperature rises.
- Line CD: Water boils at 100°C; temperature remains constant.
This shows that latent heat is used to change the state, not to increase temperature.
4. Conclusion
- Melting Point: Ice melts at 0°C, absorbing heat without a temperature change.
- Boiling Point: Water boils at 100°C, absorbing heat until it fully vaporises.
- Latent Heat: Energy is absorbed during both phase changes without a rise in temperature.
Latent Heat of Vaporization
The heat energy absorbed at constant temperature during the conversion of a liquid to gas is called the latent heat of vaporization. The specific latent heat of vaporisation is the heat required per unit mass of a liquid to change into gas. This energy is used to break intermolecular bonds rather than increasing temperature.
Effect of Atmospheric Pressure:
- The boiling point and latent heat of a substance depend on atmospheric pressure.
- Higher pressure increases the boiling point, while lower pressure decreases it.
- This principle is used in pressure cookers (higher pressure, higher boiling point, faster cooking) and mountain boiling (lower pressure, lower boiling point, slower cooking).
Melting and Boiling Points of Different Substances:
| Substance | Melting Point (°C) | Boiling Point (°C) | Specific Latent Heat of Fusion (kJ/kg) | Specific Latent Heat of Fusion (cal/g) | Specific Latent Heat of Vaporization (kJ/kg) | Specific Latent Heat of Vaporization (cal/g) |
|---|---|---|---|---|---|---|
| Water/Ice | 0 | 100 | 333 | 80 | 2256 | 540 |
| Copper | 1083 | 2562 | 134 | 49 | 5060 | 1212 |
| Ethyl Alcohol | -117 | 78 | 104 | 26 | 8540 | 200 |
| Gold | 1063 | 2700 | 144 | 15.3 | 1580 | 392 |
| Silver | 962 | 2162 | 88.2 | 25 | 2330 | 564 |
| Lead | 327.5 | 1749 | 26.2 | 5.9 | 859 | 207 |
