Topics
Relative Molecular Mass and Mole
Raoult's law and colligative properties
- Vapour Pressure of Liquid- Liquid Solutions
- Intensive property
- Extensive Property
- Ideal and Non-ideal Solutions
- Azeotropic Mixtures – Definition, Types and Examples
- Solubility of a Gas in a Liquid
- Solubility of Gases in Liquids
Dissociation- Electrolytic solute
- Dissociation- Electrolytic Solute
- Meaning of Electrolytic Solute
- Meaning of Electrolytic Solute
- Numericals
Relative molecular mass of non-volatile substances
- Relative Molecular Mass of Non-volatile Substances
- Relative Molecular Mass of Non-volatile Substances
- Determination of Relative Molecular Mass
- Determination of Relative Molecular Mass
- Depression in Freezing Point
- Freezing Point Depression
- Freezing Point Depression
- Elevation in Boiling Point Method
- Boiling Point Elevation
- Boiling Point Elevation
- Osmotic Pressure and Its Application in the Determination of Relative Molecular Mass
- Van’t Hoff- Boyle’s Law
- Van’t Hoff- Charles’ Law
- Van’t Hoff- Avogadro’s law
- Van’t Hoff
- Van’t Hoff Factor for the Electrolytes Which Dissociate and the Molecules Which Associate in Solution
- Van’t Hoff Factor
- Modification of the Formula of Colligative Properties Based on Van’t Hoff Factor
- Calculation of Degree of Dissociation and Association
- Van’t Hoff Equation and Its Interpretation
- Simple Numerical Problems on Different Methods for the Determination of Molecular Masses
- Abnormal Molecular Masses in Case of Electrolytes and in Case of Solutes Which Associate
Association
- Association
- The Meaning of Association with Respect to Dimer Formation
- Numericals
Normality, molality
- Normality, Molality, Molarity, Mole Fraction, as Measures of Concentration
- Simple Problems Relating Mass, Molar Mass and Mole
Nonvolatile, non electrolytic solute
- Nonvolatile, Non Electrolytic Solute
- Explanation of Non-volatile Solute and Non-electrolytic Solute with Examples
States of Matters: Structure and Properties Solid State
- Crystal Lattices and Unit Cells
- Relation Between Radius, Edge Length and Nearest Neighbour Distance of Atoms in a Unit Cell
- Interstitial Void
- Imperfections in Solids, Ionic, Metallic and Atomic Solids, Electrical and Magnetic Properties
- Calculations Involving Unit Cell Dimensions
- Amorphous and Crystalline Solids
- Definition of Crystal Lattice
- Definition of Unit Cell
- Types of Unit Cell
- Calculation of the Number of Atoms per Unit Cell
- Concept of Radius, Edge Length and Nearest Neighbour Distance
- Calculation of Density of Unit Cell
- Packing in 3 – D
- Voids – Types, Location, Formation
- Characteristics of Crystalline Solids;
- Point Defects – F Centers
- Electrical Properties
- Magnetic Properties
Chemical Kinetics
Order of a reaction
- Order-reaction (Meaning)
- Relation Between Order and Stoichiometric Coefficients in Balanced Equations
- Order as an Experimental Quantity
- Rate Equation for Zero Order Reaction and Its Unit
- Mathematical Derivation of Rate Equation for First Order Reaction
- Characteristics of First Order Reaction
- Definition of Half-life Period
- Derivation of Expression of Half-life Period from First Order Rate Equation
- Problems Based on First Order Rate Equation and Half Life Period
Collision Theory
- Collision Theory of Chemical Reactions
- Condition for a Chemical change
- Collisions to Be Effective
- Energy Barrier Built-up When the Collision is About to Take Place
- Activated Complex Formation
- Difference in Energy of the Reactant and the Product
Mechanism of the reaction
- Mechanism of the Reaction
- Meaning of Elementary Reaction
- Meaning of Complex and Overall Reaction
- Explanation of the Mechanism of the Reaction
- Bottleneck principle and slow step
- Relationship Between the Rate Expression, Order of Reactants and Products at the Rate- Determining Step
- Units of Rate Constant
Effect of temperature on the rate constant of a reaction
- Effect of Temperature on the Rate Constant of a Reaction
- Collision Theory of Chemical Reactions
- Meaning of the Symbols of Arrhenius Equation
- Related Graph, Evaluation of Ea and a from the Graph
- Meaning of Slope of the Graph
- Conversion from Exponential to Log Form of the Equation
- Relationship Between the Increase in Temperature and the Number of Collisions
- Numerical Based on Arrhenius Equation
The concept of energy
- The Concept of Energy
- Exothermic and Endothermic Reactions
- Concept of Energy Barrier
- Temperature Dependence of the Rate of a Reaction
- Threshold Energy
- Formation of Activated Complex
- Effect of Catalyst on Activation Energy and Reaction Rate
Catalyst
- Catalyst Defination
- Types of Catalyst
- Homogeneous and Heterogeneous Catalyst
- Elementary Treatment of Intermediate Compound Formation Theory with Examples
- Adsorption Theory
- Effect of Catalyst on the Rate of Reaction
- Characteristics of a Catalyst
- Surface Area of a Catalyst
Meaning of Chemical Kinetics
- Scope and Importance of Kinetics of the Reaction
- Slow and Fast Reactions
Rate of Reaction
- Rate of Chemical Reaction
- Representation of Rate of Reaction in Terms of Reactants and Products
- Determination of Rate of Reactions Graphically
- Instantaneous and Average Rate of Reaction
- Factors Affecting Rate of Reaction
Law of Mass Action
- Statement and Meaning of Active Mass
- Law of Mass Action
- Explanation with an Example – General Reactions
Effect of concentration of reactants on the rate of a reaction
- Effect of Concentration of Reactants on the Rate of a Reaction
- Qualitative Treatment
- Statement of Rate Law
- General rate equation
- Relation Between the Rate of the Reaction with Rate Constant with Respect to Various Reactants
Molecularity of the reaction
- Molecularity of the Reaction
- Meaning – Physical Picture
- Relation Between Order, Molecularity and the Rate of a Reaction
- Differences Between Order and Molecularity of a Reaction
Chemical Equilibria
Reversible reactions and dynamic equilibrium
- Reversible Reactions and Dynamic Equilibrium
- Characteristics of Chemical Equilibrium
- The Dynamic Nature
- Equilibrium Constant in Terms of Concentration Kc
- Gaseous Reactions
- Equilibrium Constant in Terms of Partial Pressures Kp
- Relationship Between Kp and Kc
- Characteristics of Equilibrium Constant
- Units for Equilibrium Constant
- Simple Calculations of Equilibrium Constant and Concentration
- Synthesis of Ammonia by Haber’s Proces
- Hydrolysis of Simple Esters
- The Dissociation of Dinitrogen Tetra Oxide
- The Contact Process for the Manufacture of Sulphuric Acid
Le Chatelier’s Principle and its applications to chemical equilibria
- Le Chatelier’s Principle
- Change of Concentration
- Change of Temperature
- Change of Pressure
- Effect of Catalyst
- Addition of Inert Gas
Ionic Equilibria
Arrhenius, Brönsted-Lowry and Lewis concept of acids and bases
- Arrhenius, Bronsted-lowry and Lewis Concept of Acids and Bases
Ionic product of water, pH of solutions and pH indicators
- Multistage Ionization of Acids and Bases with Examples
Salt hydrolysis
- Salt Hydrolysis
Buffer solutions
- Buffer Solutions
- Buffer Action
- Buffer Interpretations
- Buffer
Solubility product and its applications
- Solubility product
- Solubility product
Ostwald’s dilution law and its derivation
- Ostwald’s Dilution Law and Its Derivation
- Strength of Acids and Bases Based on Their Dissociation Constant
Common ion effect
- Common Ion Effect in the Ionization of Acids and Bases
- Common Ion Effect Example
Electrochemistry
Faraday’s laws of Electrolysis, Coulometer
- Faraday’S Laws of Electrolysis
- Coulometer
- Faraday’s 1st Law of Electrolysis
- Faraday’s 1st Law of Electrolysis
- Faraday’s 2nd Law of Electrolysis
- Faraday’s 2nd Law of Electrolysis
Galvanic cells, mechanism of current production in a galvanic cell;
- Primary Batteries
- Mechanism of Current Production in a Galvanic Cell
- Electrochemical Series
- Nernst Equation - Introduction
- Galvanic Cells - Introduction
- principle – oxidation reduction
- Mechanism of Production of Electric Current in a Galvanic Cell
- Measurement of potential
- Single Electrode Potentials
- Electrical Double Layer
- Standard Hydrogen Electrode
Relation between Faraday, Avogadro’s number and charge on an electron
- Relation Between Faraday, Avogadro’S Number and Charge on an Electron
Electrolytic conductance
- Electrolytic Conductance
- Specific Conductance
- Measuring of Molar and Equivalent Conductance
- Variation of Conductivity and Molar Conductivity with Concentration
- Comparison of Metallic Conductance and Electrolytic Conductance
- Relationship Between Conductance and Resistance
- Specific Resistance and Specific Conductance
- Cell Constant: Calculation of Cell Constant
- Measuring of Molar and Equivalent Conductance
- General Relationship Between
- Units, numericals, graph
- Molar Conductance of a Weak Electrolyte at a Given Concentration and at Infinite Dilution
- Kohlrausch's law
Batteries
- Batteries
- Primary and Secondary Cells
- Lead storage battery
- fuel cell
Corrosion
- Corrosion of Metals
- Mechanism of Electrochemical Reaction
- Factors Affecting Corrosion
- Prevention of Corrosion
Coordination Compounds
- Concept of Complexes
- Definitions of Some Important Terms Pertaining to Coordination Compounds
- Classification of Ligands
- Coordination Sphere
- Nomenclature of Coordination Compounds - Naming of Mononuclear Coordination Compounds
- Stereoisomerism
- Magnetic Characteristics of Coordination Compounds on the Basis of Valence Bond Theory and Crystal Field Theory
- Stability Constant
- Importance and Applications of Coordination Compounds
- Differences with a Double Salt
- calculation for a complex coordination sphere
- Study of Oxidation State of an Element in a Complex
- IUPAC rules of nomenclature of coordination compounds
- Valence Bond Theory (VBT)
- Crystal Field Theory (CFT)
- Stability of Coordination Compounds
- Isomerism in Coordination Compounds
Chemistry of p-Block Elements: Group 16, 17, 18
- Concept of Group 16 Elements
- Oxygen, Sulphur, Selenieum, Tellurium
- Ozone
- Sulphur - Allotropic Forms
- Compounds of Sulphur
- Sulphur Dioxide
- Laboratory and Industrial Preparation from Sulphites and Sulphide Ores
- Reaction of Sulphur Dioxide
- Sulphuric Acid
- Concept of Group 17 Elements
- Anomalous Behaviour of Fluorine
- Fluorine
- Reaction of Fluorine
- Chlorine
- Interhalogen Compounds
- Concept of Group 18 Elements
- State, Low Reactivity
- Formation of Xenon Compounds with Fluorine and Oxygen – Equation
- Hybridization, Shape and Structure of Compounds
- Oxoacids of Halogens
Preparation/ Manufacture, Properties and Uses of Compounds of Groups 16, 17, – Ozone, Sulphur Dioxide, Sulphuric Acid, Hydrochloric Acid
- Ozone
- Manufacture by Siemen’s Ozoniser
- Thermal Decomposition of Ozone
- Oxidising Nature
- Reaction with Lead Sulphide, Potassium Iodide and Mercury
- Ozonolysis of Ethene
- Ozone Depletion in the Stratosphere
- Hydrogen Peroxide
- Preparation from Peroxide
- Structure of Hydrogen Peroxide
- Oxidising Properties
- Reaction with Ki, Pbs, Acidified Feso4,
- Reducing Properties
- Sulphuric Acid
- Hydrochloric Acid
- Lab preparation, its acidic nature, reaction with ammonia, carbonates and sulphites, formation of aqua regia and its uses
Chemistry of Transition and Inner-Transition Elements: d-Block: 3d, 4d and 5d series f-Block: 4f and 5f series
- D-block: 3d, 4d and 5d Series
- F-block: 4f and 5f Series
- General Properties of the Transition Elements (D-block)
- The Lanthanoids
- Shielding Effect
- Radioactive Nature
- The Actinoids
- Metallurgy of Al, Zn, Fe, Cu and Ag in Terms of Equations
- Electrochemical Principles of Metallurgy
- Electrolytic Refining and Uses
- Compounds Agno3, Kmno4
- Silver Nitrate: Equation of Preparation
- Use in Laboratory and in Photography
- Potassium Permanganate
- Structure and Shape
- Equation of Extraction from Pyrolusite Ore
- Oxidising Nature in Acidic, Basic and Neutral Medium
- Use in Redox Titration
- Potassium Dichromate
- Equation of Extraction from Chromite Ore
- Structure and Shape of Molecule and Its Use in Titration
- Occurrence of Metals
Alkyl and Aryl Halides
Organometallic compounds
- Organo Metallic Compounds
- Organometallic Compounds Including Grignard’S Reagent
- Preparation and Uses
- Wilkinson’s and Ziegler-natta Catalyst
The nomenclature of aliphatic compounds containing halogen atom
- Naming the Halogen Derivatives of Alkanes by Using Common System
- IUPAC System for Mono, Di and Tri-halo Derivatives
Preparation, properties, uses of haloalkanes
- Preparation of Haloalkanes from - Alkane and Halogen
- Preparation of Haloalkanes from - Alkene and Hydrohalide
- Preparation of Haloalkanes from - Alcohol
- General properties of Haloalkanes
- Reaction of Haloalkanes with - sodium nitrite
- Reaction of Haloalkanes with - Silver Nitrite
- Reaction of Haloalkanes with - Aq. Sodium Hydroxide
- Reaction of Haloalkanes with - Alcoholic Potassium Hydroxide
- Uses of Halogen Derivatives of Alkanes in Day to Day Life and in Industry May Be Discussed
Chlorobenzene
- Chlorobenzene
- Preparation from Aniline
- Physical Properties and Chemical Properties
- Electrophillic Substitution Reactions
- Nucleophilic Substitution
- Replacement of Chlorine with -oh, -nh2
- Reduction to Benzene
- Wurtz-fittig Reaction
- Fittig Reaction
- Addition Reaction with Magnesium
- Polyhalogen Compounds
Preparation, properties, and uses of the following
- Preparation, Properties, and Uses of the Following: Ethyl Bromide, Chloroform, Iodoform, Haloform Reaction
- Haloform Reaction for the Preparation of Chloroform and Iodoform from Alcohol Should Be Discussed
Alcohols and Phenols
Methods of preparation, manufacture, properties and use
- Methods of Preparation
- Methods of Preparation of Alcohol from Hydration of Alkenes
- Methods of Preparation of Alcohol from Direct Hydration, Hydroboration Oxidation
- Methods of Preparation of Alcohol from Grignard’S Reagent
- Methods of Preparation of Alcohol from Hydrolysis of Alkyl Halides
- Methods of Preparation of Alcohol from Reduction of Carboxylic Acids
- Manufacture of Methanol by Bosch Process and Ethanol by Fermentation of Carbohydrates
- Acidity of Alcohols: Reaction with Sodium
- Esterification with Mechanism
- Reaction with Hydrogen Halides
- Alcohol Reaction with Pcl5, Pcl3 and Socl2
- Alcohol Reaction with Acid Chlorides and Acid Anhydrides
- Oxidation of Alcohol
- Mechanism of Dehydration of Alcohols
- Uses of Alcohols
Preparation, properties and uses of ethane-1, 2 diol, propane-1, 2, 3 triol
- Ethane-1, 2-diol
- Ethane-1, 2-diol Preparation from Ethene
- Physical Properties, Chemical Properties
- Oxidation to Oxalic Acid and Reaction with Hcl
- Propane – 1, 2, 3-triol
- Preparation from Soap: Saponification
- Physical Properties
- Chemical Properties
- Oxidation with Kmno4 and Reaction with Oxalic Acid
Distinction between primary, secondary and tertiary alcohols
- Distinction Through Oxidation, Dehydration and Luca's Test
- Phenols
- Preparation of Phenol from Diazonium Salt
- Preparation of Phenol from Chlorobenzene (Dow’S Process)
- Preparation of Phenol from Benzene Sulphonic Acid
- Manufacture of Phenol from Cumene
- Physical and Chemical Properties
- Acidic Character of Phenol
- Phenol Reaction with Sodium Hydroxide
- Phenol Reaction with Sodium
- Phenol Reaction with Zinc
- Phenol Reaction with Acetyl Chloride and Acetic Anhydride
- Phenol Reaction with Phosphorus Penta Chloride
- Bromination, Nitration and Sulphonation (Electrophilic Substitution Reactions)
- Chemical Properties of Phenol
- Reimer
- Tiemann Reaction Test for Phenol
- Fecl3 Test, Azo Dye Test
Classification, general formulae, structure and nomenclature
- Classification into Monohydric, Dihydric and Polyhydric Alcohols
- Structure of Alcohols
- Nomenclature
- Distinction Between Primary, Secondary and Tertiary Alcohols
- Physical and Chemical Properties
Conversion of one alcohol into another
- Conversion of One Alcohol into Another
Ethers, Carbonyl Compounds
Carbonyl compounds
- Introduction of Aldehydes, Ketones and Carboxylic Acids
- Preparation of Aldehydes and Ketones
- Preparation of Aldehydes and Ketones from Alcohol
- Preparation of Aldehydes and Ketones from Alkenes (Ozonolysis)
- Preparation of Aldehydes and Ketones from Alkynes (Hydration)
- Preparation of Aldehydes and Ketones from Calcium Salt of Carboxylic Acids
- Preparation of Aldehydes and Ketones from Nitriles (Stephen Reaction, Grignard’s Reagent)
- Preparation of Aldehydes and Ketones from Esters
- Preparation of Aldehydes and Ketones From acid chlorides
- Chemical Reactions of Aldehydes and Ketones - Nucleophilic Addition Reactions
- Reactions with Ammonia, Hydroxylamine, Hydrazine and Phenyl Hydrazine
- Oxidation Reactions
- Reduction to Alcohol and Alkanes
- Base Catalysed Reactions
- Iodoform Reaction
- Uses of Aldehydes and Ketones
- Difference Between Formaldehyde and Acetaldehyde
- Difference Between Aldehydes and Ketones
- Lab Preparation from Toluene, Oxidation by Chromyl Chloride
- Physical Properties of Benzaldehyde
- Chemical Properties of Benzaldehyde
- Oxidation and Reduction of Benzaldehyde
- Nucleophilic Addition Reaction
- Benzaldehyde Reactions with Ammonia and Its Derivatives
- Benzaldehyde Reaction with Phosphorus Pentachloride
- Cannizzaro Reaction
- Benzoin Condensation
- Bromination, Nitration and Sulphonation (Electrophilic Substitution Reactions)
- Distinction Between Aromatic and Aliphatic Aldehydes
- Uses of Benzaldehyde
- Physical Properties – State and Boiling Point
- Perkin’s Reaction
Ethers
- General Formula and Structure
- Nomenclature
- Preparation of Ethers
- Physical and Chemical Properties of ether
- Uses of Ethers
- Structure of Ethereal Group
- Preparation from alcohol (Williamson’s synthesis)
- Reaction with Chlorine
- Oxidation (Peroxide Formation)
- Ether Reaction with HI
- Ether Reaction with Pcl5
Carboxylic acids and Acid Derivatives
Carboxylic acids
- Introduction of Carboxylic Acids
- Methods of Preparation of Carboxylic Acids
- Nomenclature of Carboxylic Acids
- Physical Properties of Carboxylic Acids
- Classification of Mono and Di Carboxylic Acids with Examples
- Preparation of Carboxylic Acids from Alcohols, Aldehydes
- Preparation of Carboxylic Acids from Nitriles
- Preparation of Carboxylic Acids from Grignard’S Reagent
- Acidic Character: Reaction with Active Metals, Alkalies, Carbonates and Bicarbonates
- Formation of Acid Derivatives
- Decarboxylation
- HVZ Reactions
- Tests for Acids: Formic Acid and Acetic Acid
- Uses of formic acid and acetic acid
- Oxalic Acid: Preparation from Glycol and Sodium Formate
- Oxalic Acid Reaction with Alkali
- Esterification Reaction
- Oxalic Acid Reaction with Pcl5
- Action of Heat on Oxalic Acid
- Oxidation by Potassium Permanganate
- Chemical Properties Oxalic Acid
- Physical Properties Oxalic Acid
- Test for Oxalic Acid
- Uses of Oxalic Acid
- Benzoic acid Preparation from benzaldehyde and Toluene
- Physical Properties of Benzoic Acid
- Chemical Properties of Benzoic Acid
- Benzoic Acid Reaction with Sodium Hydroxide, Sodium Carbonate
- Esterification reaction
- Benzoic Acid Reaction with Phosphorus Pentachloride
- Decarboxylation
- Substitution of Benzene Ring (Meta Directive Effect of Carboxylic Acid Group) Nitration and Sulphonation
- Test for Benzoic Acid
- Uses of Benzoic Acid
Acid derivatives
- Formation of Acid Derivatives
- Laboratory Preparation of Acid Derivatives
- Properties and Uses of
- Urea Preparation (By Wohler'S Synthesis)
- Properties and Uses of Urea
- Manufacture of Urea from Ammonia and by Cyanamide Process
- Acid Derivatives:General and Structural Formula
- Acid Derivatives: IUPAC Nomenclature
- Laboratory Preparation and Uses of
- Manufacture of Urea from Ammonia and by Cyanamide Process
- Acetyl Chloride Hydrolysis
- Acetylation of Alcohol, Ammonia and Amines
- Rosenmund’s Reduction
- Formation of Acetic Anhydride
- Reaction with Grignard Reagent
- Acetic Anhydride - Hydrolysis
- Acetylation of Ethanol and Aniline
- Acetic Anhydride - Reaction with PCl5
- Acetamide - Acid Hydrolysis
- Acetic Anhydride - Reaction with Alkalies
- Hoffmann’s Degradation
- Acetamide Reaction with Nitrous Acid
- Acetamide Dehydration
- Acetamide Reduction
- Amphoteric Nature (Reaction with Hcl and Reaction with Hgo)
- Ethyl Acetate - Acid Hydrolysis
- Ethyl Acetate - Saponification
- Ethyl Acetate Reaction with Ammonia
- Ethyl Acetate Reaction with Phosphorus Penta Chloride
- Ethyl Acetate Reduction
- Urea - Hydrolysis
- Salt Formation with Nitric Acid
- Urea -biuret Reaction (Test)
- Reaction with Hot Sodium Hydroxide (Formation of Ammonia and Carbon Dioxide).
- Preparation of Aldehydes
Cyanides, Isocyanides, Nitro compounds, Amines and Diazonium Salt
- Cyanides, Isocyanides and Nitro Compounds
- Methods of Preparation: Cyanides from Alkyl Halide
- Methods of Preparation Cyanide - from Amide
- Methods of Preparation Isocyanides from Alkyl Halide
- Methods of preparation From primary amines
- Methods of Preparation Nitro Compounds from Alkyl Halide
- Methods of Preparation Nitro Compounds from Primary Amines
- Physical Properties Nitro Compounds
- Chemical properties Nitro compounds
- Cyanides and Isocyanides - Hydrolysis
- Cyanides and Isocyanides - Reduction
- Nitro Compounds - Reduction in Acidic and Neutral Medium
- Cyanides and Isocyanides Uses
- Nitrobenzene Method of Preparation
- Physical Properties of Nitrobenzene
- Chemical Properties of Nitrobenzen
- Electrophilic Substitution (Chlorination and Nitration)
- Meta Substitution
- Uses of Nitrobenzene
- Introduction of Amines
- Nomenclature of Animes
- Classification of Amines
- Method of Preparation Amines from Alcohol
- Preparation of Amines
- Method of Preparation Amines from Cynides
- Method of Preparation Amines from amides
- Method of Preparation Amines from Nitro - Compounds
- Physical Properties of Amines
- Chemical Reactions of Amines - Basic Character of Amines
- Chemical Reactions of Amines - Alkylation and Acylation
- Chemical Reactions of Amines - Reaction with Nitrous Acid
- Distinction Between Primary, Secondary and Tertiary Amines
- Aniline
- Aniline Method of Preparation
- Physical Properties Anilines
- Chemical Properties Anilines
- Anline Reaction with HCl and H2so4
- Acetylation, Alkylation
- Benzoylation
- Chemical Reactions of Amines - Carbylamine Reaction
- Diazotisation
- Chemical Reactions of Amines - Electrophilic Substitution
- Test for Aniline
- Uses of Aniline
- Introduction of Diazonium Salts
- Method of Preparation of Diazonium Salts
- Importance in Synthesis of Other Organic Compounds
- Sandmeyer’s Reaction
- Gattermann Reaction and Balz – Scheimann Reaction
- Properties of Diazonium Salts
Polymers
- Polymerisation
- The Principle of Addition and Condensation Polymerisation
- Thermoplastics
- Thermosetting Plastics
- Chemotrophs
- Physical Properties of Polymers
- Polythene
- Polypropene
- PVC
- PTFE
- Polystyrene
- Types of Polymerisation Reactions - Rubber
- Nylon 66
- Nylon 6
- Types of Polymerisation Reactions - Condensation Polymerisation Or Step Growth Polymerisation
- Uses of Polymer
Biomolecules – carbohydrates, proteins, enzymes, vitamins and nucleic acids
- Introduction of Carbohydrates
- Carbohydrates classification
- D-L configuration
- Structures of Glucose
- Introduction of Proteins
- Amino Acids
- General Structure Amino Acid
- Classification and Zwitter Ion Formation
- Isoelectric Point
- Classification of Proteins on the Basis of Molecular Shape
- Denaturation of Proteins
- Primary and Secondary Structures of Proteins
- Enzymes
- Mechanism of Enzymatic Action
- Introduction of Vitamins
- Vitamin Deficiency Diseases
- Introduction of Nucleic Acids
- basic unit – purine and pyrimidine
Chemistry in Everyday Life
- Chemicals in Medicines
- Chemicals in Food
- Soaps and Detergents
- Therapeutic Action of Different Classes of Drugs - Antimicrobials
Surface Chemistry
- Difference Between Absorption and Adsorption
- Factors Affecting Adsorption of Gases on Solids
- Colloidal State
- Properties of Colloidal Solutions
- Various Application of Colloids
- Coating with a Protective Layer
- Metal Plating (Galvanization, Tinning, Electroplating)
- Anodization (Formation of Oxide Layer)
- Alloying (Corrosion-Resistant Alloys)
Coating with a Protective Layer
Corrosion is a natural process that deteriorates metals due to environmental factors like moisture, oxygen, and chemicals. To protect metals, especially iron, various methods are used to prevent rusting and extend the lifespan of metal objects. The rate of corrosion can be reduced by isolating metals from direct contact with air and moisture.
A layer of paint, oil, grease, varnish, or enamel is applied to block air and moisture from reaching the metal surface.
- Painting and Varnishing: Metals like iron gates, railings, and furniture are coated with paint or varnish to prevent rust. However, if the paint gets scratched, rusting starts underneath.
- Greasing and Oiling: It is used for moving parts of machines, gears, and bicycle chains to prevent rusting by reducing moisture contact.
- Enamelling: A glassy layer fused onto metals to provide long-term corrosion resistance.
Limitations:
- If the coating gets scratched or damaged, rusting can begin underneath.
- It requires regular maintenance and reapplication over time.
Metal Plating (Galvanisation, Tinning, Electroplating)
A non-corrodible metal is coated onto corrodible metals like iron or steel to prevent corrosion.
1. Galvanisation: A thin layer of zinc is applied to iron or steel to prevent rusting. Examples include shiny iron nails and pins. Zinc corrodes before iron because it is more electropositive, but once the zinc layer wears off, iron starts rusting.
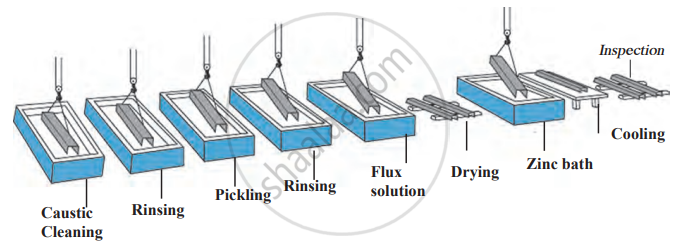
Galvanising Process
2. Tinning: Molten tin is coated on brass or copper utensils (also called kalhaee) to prevent corrosion. This protects against the greenish poisonous layer that forms on copper and brass, especially when in contact with acidic foods like buttermilk or curry.
3. Electroplating: A less reactive metal (silver, gold, or chromium) is deposited onto a more reactive metal using electrolysis. Examples include silver-plated spoons, gold-plated jewellery, and chromium-plated taps.
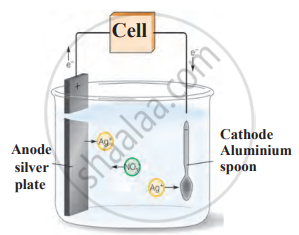
Electroplating
Limitations:
- Over time, the protective layer wears off, exposing the metal to corrosion.
- The plating process can be expensive.
Anodisation (Formation of Oxide Layer)
In anodisation, metals like aluminium and copper are coated with a thin and strong oxide layer using electrolysis.
- The oxide layer is uniform, strong, and durable, preventing further oxidation.
- Protection can be increased by making the oxide layer thicker.
Example: Anodised aluminium cookware forms a protective Al₂O₃ layer, preventing oxidation and corrosion.
Limitations: Only applicable to certain metals like aluminium and copper.

Anodization
Alloying (Corrosion-Resistant Alloys)
Metals are mixed with other metals or nonmetals to create alloys, which are stronger and more resistant to corrosion.
- It provides permanent protection against corrosion.
- Improves the strength and durability of metals.
Examples,
- Bronze (90% copper + 10% tin): Used for statues and sculptures, resistant to sun and rain.
- Stainless Steel (74% iron + 18% chromium + 8% carbon): Does not rust or stain, making it ideal for utensils, construction materials, and surgical instruments.
- Coins made from alloys: Modern coins are made from corrosion-resistant alloys to prevent wear and tear.
Limitations: Expensive and difficult to separate metals once alloyed.

Coins made from various alloys
