Advertisements
Advertisements
Question
Explain the meaning of the term latent heat. State its S. I. unit.
Solution
The heat absorbed or liberated by a substance during its change of state at a constant temperature is called latent heat. Its S. I. unit is J/kg.
APPEARS IN
RELATED QUESTIONS
State two factors upon which the rate of emission of thermions depends.
A refrigerator converts 100g of water at 20℃ to ice at – 10℃ in 73.5 min. Calculate the average rate of heat extraction in watt. The specific heat capacity of water is 4.2 J kg-1 K-1, specific latent heat of ice is 336 J g-1 and the specific heat capacity of ice is 2.1 J kg-1 K-1.
Calculate the total amount of heat energy required to convert 100 g of ice at −10℃ completely into water at 100℃. Specific heat capacity of ice = 2.1 J g-1 K-1, specific heat capacity of water = 4.2 J g-1K-1, specific latent heat of ice = 336 J g-1.
During transformation of liquid phase to solid phase, the latent heat is ______.
Answer the following:
Explain the role of latent heat in the change of state of a substance.
Explain the following temperature vs time graph.
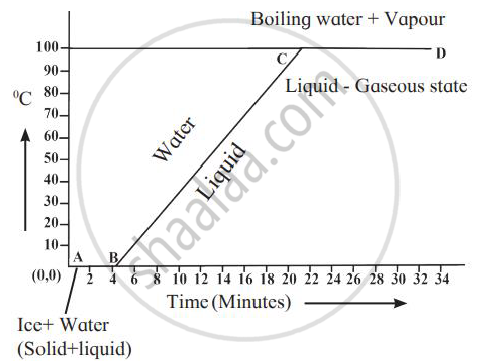
Define the following terms:
(i) Latent heat,
(ii) Latent heat of fusion of ice.
Name two factors on which the heat absorbed or given out by a body depends.
What happens to the heat supplied to a substance when the heat supplied causes no change in the temperature of the substance?
When 1 g of ice at 0 °C melts to form 1 g of water at 0 °C then, is the latent heat absorbed by the ice or given out by it?
What do you understand by the ‘latent heat of vaporization’ of a substance?
Explain the meaning of greenhouse effect.
What observation you will record and how will you determine the specific latent heat of fusion of ice?
Calculate the total amount of heat required to convert 100g ice at 0°C to steam at 100°C.
(Specific latent heat of fusion of ice = 336 J/g, specific latent heat of vaporization of steam = 2260 J/g, specific heat capacity of water = 4.2 J/g°C).
If pressure increases, the melting point of a substance ______.
Find the odd one out and give its explanation.
Specific latent heat L = ______.
Give some practical applications of specific latent heat of ice.
2875 J of heat is required to melt 115 g of lead at its melting point. Calculate the specific latent heat capacity of fusion of lead.
