Advertisements
Advertisements
Question
What observation you will record and how will you determine the specific latent heat of fusion of ice?
Solution
A clean and dry copper calorimeter with a strirrer, is weighed when empty, again weighed when filled nearly 2/3 with water. Then initial temperature is noted. Some pieces of dry ice are transferred into the calorimeter. The calorimeter is placed inside the box and is continuously stirred till the whole of ice is melted. The final temperature is carefully noted. Then the mass of calorimeter with water and ice is again weighed.
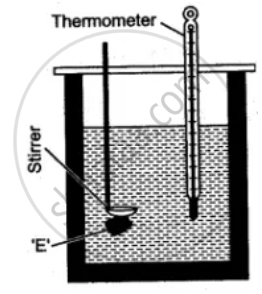
The observations recorded are:
Mass of empty calorimeter with stirrer = m1 gm.
Mass of calorimeter + water = m2 gm.
Initial temperature of water = t1 °C
Final temperature of mixture = t2 °C
Mass of calorimeter + Water + Ice (in the form of water) = m3 gm.
Temperature of ice = 0 °C
If L j/gm be the specific latent heat of fusion of ice and sc be the specific heat capacity of copper, then:
Mass of ice method = (m3 - m1) gm
Heat required by ice water at 0 °C to rise to t2 °C
= (m3 - m2) × 4.2 × (t2 - 0)
Heat lost by calorimeter = m1 × Sc × (t1 - t2)
Heat lost by water = (m2 - m1) × 4.2 × (t1 - t2)
From the heat equation,
Heat gained = Heat lost
(m3 - m2) L + (m3 - m2) × 4.2 × t2 = m1 × sc (t1 - t2) + (m2 - m1) × 4.2 × (t1 - t2)
Therefore,
L = `[(("m"_1"s"_"c")("t"_1 - "t"_2) + ("m"_2 - "m"_1) xx 4.2 xx ("t"_1 - "t"_2) - ("m"_3 - "m"_2) xx 4.2 xx "t"_2)/(("m"_3 - "m"_2))]`
APPEARS IN
RELATED QUESTIONS
State two factors upon which the rate of emission of thermions depends.
What is the Greenhouse effect?
Explain the following:
The surrounding become pleasantly warm when water in a lake starts freezing in cold countries.
Explain the following:
The heat supplied to a substance during it change of state, does not cause any rise in its temperature.
Liquid ammonia is used in ice factory for making ice from water. If water at 20°C is to be converted into 2 kg ice at 0°C, how many grams of ammonia are to be evaporated? (Given: The latent heat of vaporization of ammonia = 341 cal/g)
Water expands on reducing its temperature below ______°C.
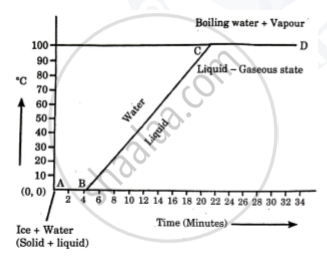
Explain the following temperature Vs. time graph:
Define the following terms:
(i) Specific latent heat,
(ii) Specific latent heat of fusion.
A substance changes from its solid state to the liquid state when heat is supplied to it. What name is given to heat absorbed by the substance.
When 1 g of ice at 0 °C melts to form 1 g of water at 0 °C then, is the latent heat absorbed by the ice or given out by it?
Why water get cooled in a ‘Surahi’ in hot season?
Why do we feel much comfortable when we sit under a moving fan especially when our body is sweating?
Explain, why no tracks are left on the ice during ice skating?
State the main precautions to be taken in finding the latent heat of steam.
If there is no Heat loss to the surroundings, the heat released by the condensation of m1 g of steam at 100°C into water at 100°C can be used to convert m2 g of ice at 0°C into water at 0°C.
(i) Find:
(a) The heat lost by steam in terms of m1
(b) The heat gained by ice in terms of m2
(ii) Form a heat equation find the ratio of m2 : m1
Specific latent heat of vaporization of steam = 2268 kJ/kg
Specific latent heat of fusion of ice = 336 kJ/kg
Specific heat capacity of water = 4200 J/kg°C
Write scientific reason.
Use a pressure cooker to cook food in cold air.
Who introduced the term latent heat?
Calculate the amount of heat required to convert 200g of ice at 0°C into the water at 0°C Specific latent heat of fusion of ice = 336 Jg-1
Specific latent heat of a substance ______.
Observe the following graph and answer the following questions:

- What does the graph represent?
- What does the line AB represent?
- What does the line BC represent?
