Topics
Gravitation
- Concept of Gravitation
- Force
- Motion and Rest
- Centripetal Acceleration and Centripetal Force
- Kepler’s Laws
- Newton’s Universal Law of Gravitation
- Uniform Circular Motion (UCM)
- Earth’s Gravitational force
- Acceleration Due to Gravity (Earth’s Gravitational Acceleration)
- Concept of Mass and Weight
- Gravitational Waves
- Free Fall
- Gravitational Potential Energy
- Weightlessness in Space
Periodic Classification of Elements
- History of Periodic Table: Early Attempts at the Classification of Elements
- Dobereiner’s Triads
- Newland's Law of Octaves
- Mendeleev’s Periodic Table
- Merits and Demerits of Mendeleev’s Periodic Table
- Modern Periodic Law
- The Modern Periodic Table
- Structure of the Modern Periodic Table
- Modern Periodic Table and Electronic Configuration of Elements
- Groups and Electronic Configuration
- Periods and Electronic Configuration
- Periodic Properties
- Valency
- Atomic Radius Or Atomic Size
- Metallic and Non-metallic Characters
- Group VIIA Or Group 17 (The Halogens)
Chemical Reactions and Equations
- Chemical Reaction
- Chemical Equation
- Balancing Chemical Equation
- Types of Chemical Change or Chemical Reaction
- Direct Combination (or Synthesis) Reaction
- Decomposition Reactions
- Single Displacement Reactions
- Double Displacement Reaction
- Energy Change in Chemical Reactions
- Rate of Chemical Reaction
- Factors Affecting the Rate of a Chemical Reaction
- Oxidation, Reduction and Redox Reactions
- Corrosion of Metals
- Rancidity of Food and Its Prevention
Effects of Electric Current
- Electric Circuit
- Ohm's Law (V = IR)
- Heating Effect of Electric Current
- Magnetic Effect of Electric Current
- Right-hand Thumb Rule
- Magnetic Field Due to Current in a Loop (Or Circular Coil)
- Magnetic Field Due to a Current Carving Cylindrical Coil (or Solenoid)
- Force on a Current Carrying Conductor in a Magnetic Field
- Fleming’s Left Hand Rule
- Electric Motor
- Electromagnetic Induction
- Galvanometer
- Fleming’s Right Hand Rule
- Types of Current
- Electric Generator
Heat
Refraction of Light
Lenses
- Concept of Lenses
- Spherical Lens
- Convex Lens
- Images Formed by Convex Lenses
- Concave Lens
- Images Formed by Concave Lenses
- Sign Convention
- Lens Formula
- Magnification Due to Spherical Lenses
- Power of a Lens
- Combination of Lenses
- Human Eye
- Working of the Human Eye
- Eye Defect and Its Correction: Myopia Or Near-sightedness
- Eye Defect and its Correction: Hypermetropia or Far-sightedness
- Eye Defect and Its Correction: Presbyopia
- Persistence of Vision
Metallurgy
- Types of Element: Metals
- Physical Properties of Metals
- Chemical Properties of Metal
- Reactions of Metal
- Reactivity Series of Metals
- Types of Element: Non-metal
- Physical Properties of Non-metal
- Chemical Properties of Non-metal
- Ionic Compounds
- Metallurgy
- Basic Principles of Metallurgy
- Extraction of Reactive Metals
- Extraction of Aluminium
- Extraction of Moderately Reactive Metals
- Extraction of Less Reactive Metals
- Refining of Metals
- Corrosion of Metals
- Prevention of Corrosion
Carbon Compounds
- Carbon Compounds in Everyday Life
- Bonds in Carbon Compounds
- Carbon: a Versatile Element
- Properties of Carbon
- Hydrocarbons
- Structural Variations of Carbon Chains in Hydrocarbons
- Functional Groups in Carbon Compounds
- Homologous Series of Carbon Compound
- Nomenclature of Organic Compounds
- The IUPAC System of Nomenclature
- Chemical Properties of Carbon Compounds
- Ethanol
- Ethanoic Acid
- Macromolecules and Polymers
Space Missions
- Concept of Space Missions
- Artificial Satellites
- Types of Satellite
- Orbits of Artificial Satellites
- Space launch technology
- Space Missions Away from Earth
- India’s Space Programmes: Chandrayaan – 1
- India’s Space Programmes: Chandrayaan – 2
- India’s Space Programmes: Chandrayaan – 3
- India’s Space Programmes: Mangalyaan (Mars vehicle)
- India’s Space Programmes: Missions to Other Planets
- India and Space Technology
- Space Debris and Its Management
School of Elements
The Magic of Chemical Reactions
The Acid Base Chemistry
- Properties of Acids
- Strength of Acidic or Basic Solutions
- Strength of Acidic or Basic Solutions
- Acids, Bases and Their Reactivity
- Acid or a Base in a Water Solution
- Preparation and Uses of Baking Soda
- Preparation and Uses of Bleaching Powder
- Preparation and Uses of Washing Soda
- Preparation and Uses of Plaster of Paris
- Chemicals from Common Salt - Soap as a Salt
The Electric Spark
All about Electromagnetism
- Magnetic Force
- The Bar Magnet
- Right-hand Thumb Rule
- Magnetic Field Due to Current in a Loop (Or Circular Coil)
- Magnetic Field Due to a Current Carving Cylindrical Coil (or Solenoid)
- Force on a Current Carrying Conductor in a Magnetic Field
- Electric Motor
- Electromagnetic Induction
- Alternating Current (A.C.) Generator
- Direct Current Motor
- Household Electrical Circuits
Wonders of Light 1
- Spherical Mirrors
- Concave Mirror
- Concave Mirror
- Sign Convention
- Linear Magnification (M) Due to Spherical Mirrors
- Images Formed by Sperical Lenses
- Convex Lens
- Sign Convention
- Magnification Due to Spherical Lenses
- Power of a Lens
- Human Eye
- Eye Defect and Its Correction: Myopia Or Near-sightedness
- Spherical Mirrors
Wonders of Light 2
Striving for better Environment 1
- Pollution and Its Types
- Air Pollution and Its Causes
- Effects of Air Pollution
- Water Pollution and Its Causes
- Effects of Water Pollution
- Soil Pollution and its Causes
- Effects of Soil Pollution
- Noise Pollution
- Radioactive Pollution and Effects
- Abatement of Pollution
- Sustainable Use of Resources
- Properties and Forms of Carbon
- Carbon Compounds and Their Uses
Properties and Forms of Carbon
Carbon is a fundamental element present in all living organisms and many non-living substances. Organic compounds contain carbon, except for a few inorganic carbon compounds like carbonates, bicarbonates, and oxides of carbon.
- Atomic number: 6
- Atomic mass: 12.01 g/mol
- Found in both free (coal, graphite) and combined states (carbonates, hydrocarbons, CO₂).
- Forms bonds with elements like hydrogen, oxygen, chlorine, and sulphur, creating various compounds.
Catenation Property of Carbon:
Carbon has a unique ability to form long chains and rings by bonding with itself. This property is called catenation. Additionally, carbon can form double and triple bonds with elements like oxygen and nitrogen, leading to diverse organic compounds.
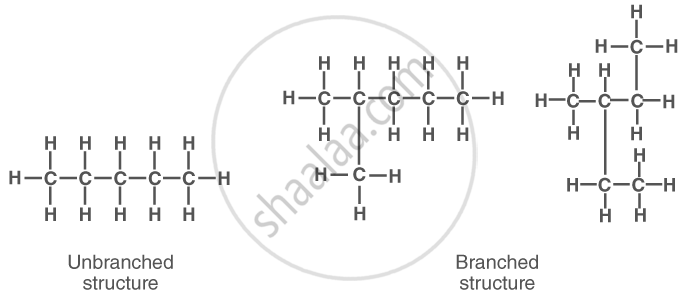
Allotropes of Carbon:
Carbon exists in multiple forms called allotropes, which have different physical properties but similar chemical behaviour.
|
Diamond: the hardest known material, used in jewellery and cutting tools. |
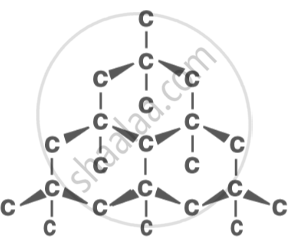 |
|
Graphite: a good conductor of electricity, used in pencils and lubricants. |
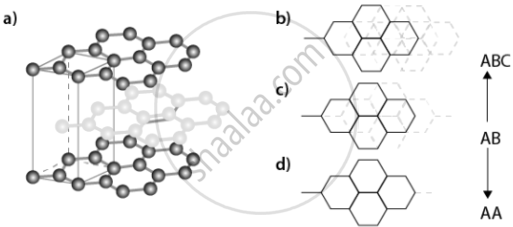 |
|
Graphene: a single-layer structure with high electrical conductivity. |
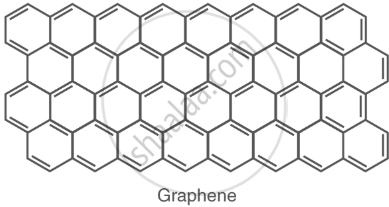 |
|
Fullerenes (C₆₀): spherical molecules used in nanotechnology. |
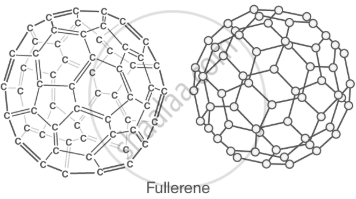 |
|
Carbon nanotubes: cylindrical structures with high strength and conductivity. |
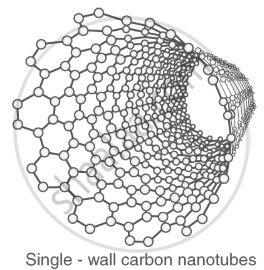 |
Carbon Compounds and Their Uses
Types of Carbon-Based Compounds
- Hydrocarbons: carbon and hydrogen compounds (e.g., methane CH₄, ethane C₂H₆).
- Carbon-Oxygen Compounds: Includes CO₂, CO, and CO₃.
- Carbon-Sulphur Compounds: Examples include CS₂ and OCS.
- Carbon-Nitrogen Compounds: Includes Cyanogen (CN)₂ and Hydrogen Cyanide (HCN).
- Carbon-Halide Compounds: Examples: carbon tetrachloride (CCl₄), carbon tetrafluoride (CF₄).
Uses of Carbon and Its Compounds
- Life’s building block: essential for all living organisms.
- Fuels: Hydrocarbons are the primary energy source.
- Polymers: Used in plastics like polyethylene and polypropylene.
- Dyes and drugs: Carbon compounds are used in pharmaceuticals.
- Diamond: Used in jewellery and industrial cutting tools.
- Graphite: Used in pencils, lubricants, and electrodes.
- Glucose: A vital energy source for living cells.
