Advertisements
Advertisements
Question
Solution
APPEARS IN
RELATED QUESTIONS
The S.I. unit of specific latent heat is ______.
The specific latent heat of fusion of water is ______.
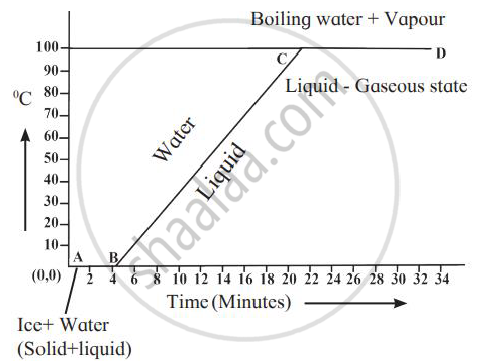
Explain the following temperature vs time graph.

Answer the following:
Explain the role of latent heat in the change of state of a substance.
Liquid ammonia is used in ice factory for making ice from water. If water at 20°C is to be converted into 2 kg ice at 0°C, how many grams of ammonia are to be evaporated? (Given: The latent heat of vaporization of ammonia = 341 cal/g)
Explain the following temperature vs time graph.
Water expands on reducing its temperature below ______°C.
Why water get cooled in a ‘Surahi’ in hot season?
Explain the meaning of greenhouse effect.
State two advantages of the high specific latent heat capacity of steam, which is about 226 × 104 J/kg?
Calculate the total amount of heat required to convert 100g ice at 0°C to steam at 100°C.
(Specific latent heat of fusion of ice = 336 J/g, specific latent heat of vaporization of steam = 2260 J/g, specific heat capacity of water = 4.2 J/g°C).
If there is no Heat loss to the surroundings, the heat released by the condensation of m1 g of steam at 100°C into water at 100°C can be used to convert m2 g of ice at 0°C into water at 0°C.
(i) Find:
(a) The heat lost by steam in terms of m1
(b) The heat gained by ice in terms of m2
(ii) Form a heat equation find the ratio of m2 : m1
Specific latent heat of vaporization of steam = 2268 kJ/kg
Specific latent heat of fusion of ice = 336 kJ/kg
Specific heat capacity of water = 4200 J/kg°C
1 kg of water is contained in a 1.25 kW kettle. Assuming specific heat capacity of water = 4.2 J/g °C and specific latent heat of vaporization = 2260 J/g, calculate:
(i) the time taken for the temperature of water to rise from 25°C to its boiling point,
(ii) the mass of water which evaporates per minute from the boiling water.
Specific latent heat of vaporisation : J/kg : : specific heat : _______
1 kg of dry air at a temperature of 40 °C can hold a maximum of 49 g of water vapour.
Write scientific reason.
Use a pressure cooker to cook food in cold air.
Calculate the amount of heat required to convert 200g of ice at 0°C into the water at 0°C Specific latent heat of fusion of ice = 336 Jg-1
The diagram below shows a cooling curve for a substance:
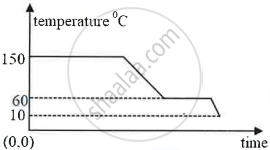
- State the temperatures at which the substance condenses.
- The temperature range in which the substance is in liquid state.
- Why do we prefer ice to ice-cold water for cooling a drink?
