Advertisements
Advertisements
Question
1 kg of water is contained in a 1.25 kW kettle. Assuming specific heat capacity of water = 4.2 J/g °C and specific latent heat of vaporization = 2260 J/g, calculate:
(i) the time taken for the temperature of water to rise from 25°C to its boiling point,
(ii) the mass of water which evaporates per minute from the boiling water.
Solution
(i) Heat required for the temperature of 1 kg (= 1000 g) of water to rise from 25°C to its boilling point (i.e. 100°C)
= Mass × Specific heat capacity × Rise in temperature
= 1000 × 4.2 × (100 - 25) = 31500 J
Power supplied by the kettle = 1.25 kW = 1.25 × 1000 W = 1250 W
Since Power = `"Energy"/"Time"`
`therefore 1250 = 31500/"t"`
Hence time taken t = `315000/1250 = 252"s"` (or 4 min 12 s)
(ii) Energy supplied by the kettle in 1 minute (= 60s) = Power × Time
= 1250 × 60 = 75000 J
Heat required for boiling water to evaporate
= Mass × Specific latent heat of vaporization
= m × 2260 = 2260 mJ
Thus 2260 m = 75000
or mass of water evaporated per minute, m = `75000/2260 = 33.18 "g"`
APPEARS IN
RELATED QUESTIONS
State any two measures to minimize the impact of global warming.
What do you understand by the term latent heat?
Explain the following:
The heat supplied to a substance during it change of state, does not cause any rise in its temperature.
A molten metal of mass 150 g is kept at its melting point 800℃. When it is allowed to freeze at the same temperature, it gives out 75,000 J of heat energy.
- What is the specific latent heat of the metal?
- If the specific heat capacity of metal is 200 J kg-1 K-1, how much additional heat energy will the metal give out in cooling to -50℃?
A refrigerator converts 100g of water at 20℃ to ice at – 10℃ in 73.5 min. Calculate the average rate of heat extraction in watt. The specific heat capacity of water is 4.2 J kg-1 K-1, specific latent heat of ice is 336 J g-1 and the specific heat capacity of ice is 2.1 J kg-1 K-1.
During transformation of liquid phase to solid phase, the latent heat is ______.
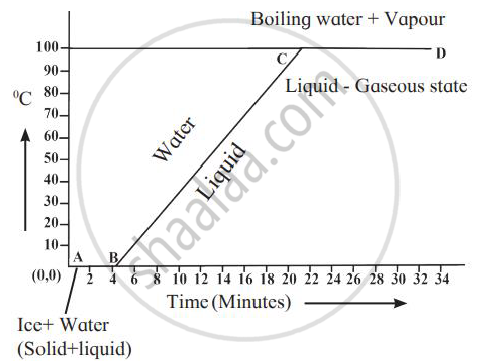
Explain the following temperature vs time graph.
Water expands on reducing its temperature below ______°C.
Name two factors on which the heat absorbed or given out by a body depends.
What observation you will record and how will you determine the specific latent heat of fusion of ice?
Calculate the total amount of heat required to convert 100g ice at 0°C to steam at 100°C.
(Specific latent heat of fusion of ice = 336 J/g, specific latent heat of vaporization of steam = 2260 J/g, specific heat capacity of water = 4.2 J/g°C).
If there is no Heat loss to the surroundings, the heat released by the condensation of m1 g of steam at 100°C into water at 100°C can be used to convert m2 g of ice at 0°C into water at 0°C.
(i) Find:
(a) The heat lost by steam in terms of m1
(b) The heat gained by ice in terms of m2
(ii) Form a heat equation find the ratio of m2 : m1
Specific latent heat of vaporization of steam = 2268 kJ/kg
Specific latent heat of fusion of ice = 336 kJ/kg
Specific heat capacity of water = 4200 J/kg°C
Define boiling point of a liquid.
Write scientific reason.
Even if boiling water is constantly heated, its temperature does not rise.
Write scientific reason.
Use a pressure cooker to cook food in cold air.
For the same mass of ice and ice-cold water, why does ice produce more cooling than ice-cold water?
Specific latent heat of a substance ______.
Calculate the total amount of heat energy required to melt 200 g of ice at 0°C to water at 100°C. (Specific latent heat of ice = 336 Jg-1, specific heat capacity of water = 4.2 Jg-1 °C-1)
