Advertisements
Advertisements
प्रश्न
The values of colligative properties of colloidal solution are of small order in comparison to those shown by true solutions of same concentration because of colloidal particles ______.
विकल्प
exhibit enormous surface area.
remain suspended in the dispersion medium.
form lyophilic colloids.
are comparatively less in number.
उत्तर
The values of colligative properties of colloidal solution are of small order in comparison to those shown by true solutions of same concentration because of colloidal particles are comparatively less in number.
Explanation:
Collodial particles are bigger aggregative than the number of particles in a collodial solution. Hence, the values of colligative are of small order as compared to values shown by true solutions at same concencration.
APPEARS IN
संबंधित प्रश्न
Define the following term:
Zeta potential
Out of MgCl2 and AlCl3, which one is more effective in causing coagulation of negatively charged sol and why?
Explain what is observed Electric current is passed through a colloidal sol?
What happens when an emulsion is centrifuged?
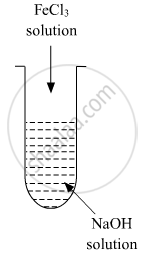
A colloidal sol is prepared by the given method in the figure. What is the charge on hydrated ferric oxide colloidal particles formed in the test tube? How is the solution represented?

A colloidal sol is prepared by the given method in the figure. What is the charge on hydrated ferric oxide colloidal particles formed in the test tube? How is the sol represented?
Assertion: Coagulation power of Al3+ is more than Na+.
Reason: Greater the valency of the flocculating ion added, greater is its power to cause precipitation (Hardy Schulze rule).
Gold number of protective colloids A, Band Dare 0.50, 0.01 and 0.10 and 0.005. The correct order of their protecting power is
What is Electrophoresis?
The conditions given below are in the context of observing Tyndall effect in colloidal solutions:
- The diameter of the colloidal particles is comparable to the wavelength of light used.
- The diameter of the colloidal particles is much smaller than the wavelength of light used.
- The diameter of the colloidal particles is much larger than the wavelength of light used.
- The refractive indices of the dispersed phase and the dispersion medium are comparable.
- The dispersed phase has a very different refractive index from the dispersion medium.
Choose the most appropriate conditions from the options given below:
