Advertisements
Advertisements
प्रश्न
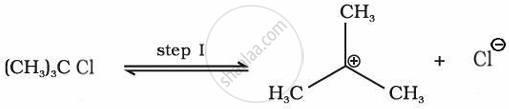
Various isomeric haloalkanes with the general formula \[\ce{C4H9Cl}\] undergo hydrolysis reactions. Among them, compound “A” is the most reactive through SN1 mechanism. Identify “A” citing the reason for your choice. Write the mechanism for the reaction.
रासायनिक समीकरण/संरचनाएँ
कारण बताइए
उत्तर
“A” is \[\ce{(CH3)3CCl}\], the carbocation intermediate obtained in tertiary alkyl halide is most stable, making A most reactive of all possible isomers.

shaalaa.com
क्या इस प्रश्न या उत्तर में कोई त्रुटि है?
