Advertisements
Advertisements
प्रश्न
What is the colour of ferrous sulphate crystals? How does this colour change after heating?
उत्तर
The colour of ferrous sulphate crystal is green. After heating, the ferrous sulphate crystal loses water molecules and forms anhydrous ferrous sulphate, which is white in colour. Subsequently, it decomposes to give ferric oxide, which is brown in colour, sulphur dioxide and sulphur trioxide.
APPEARS IN
संबंधित प्रश्न
Taking into consideration the relationship in the first pair, complete the second pair
2H2 + O2 → 2H2O :Combination Reaction :: 2HgO → 2Hg + O2:_________
Give one example of a decomposition reaction which is carried out with electricity.
What type of chemical reaction take place when silver bromide is exposed to sunlight?
Which of the following can be decomposed by the action of light?
(a) NaCl
(b) KCl
(c) AgCl
(d) CuCl
When a green iron salt is heated strongly, its colour finally changes to brown and odour of burning sulphur is given out.
(a) Name the iron salt.
(b) Name the type of reaction that takes place during the heating of iron salt.
(c) Write a chemical equation for the reaction involved.
Explain the following type of chemical reaction, giving two examples for it:
Decomposition reaction
Identify the type of following reaction :
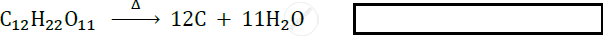
Explain the reaction given in the figure.

A metal nitrate 'A' on heating gives a metal oxide along with evolution of a brown coloured gas 'B' and a colourless gas, which helps in burning. Aqueous solution of 'A' when reacted with potassium iodide forms a yellow precipitate.
- Identify 'A' and 'B'
- Name the types of the reactions involved in the above statement.
Write the molecular formula of calcium carbonate.
