Advertisements
Advertisements
प्रश्न
Give one example of a decomposition reaction which is carried out with electricity.
उत्तर
Example of the decomposition reaction are as follows:
with electricity:
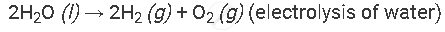
APPEARS IN
संबंधित प्रश्न
Taking into consideration the relationship in the first pair, complete the second pair
2H2 + O2 → 2H2O :Combination Reaction :: 2HgO → 2Hg + O2:_________
What type of chemical reaction is used to extract metals from their naturally occurring compounds like oxides or chlorides?
What type of chemical reaction is represented by the following equation?
X → Y + Z
A metal salt MX when exposed to light splits up to form metal M and a gas X2. Metal M is used in making ornaments whereas gas X2 is used in making bleaching powder. The salt MX is itself used in black and white photography.
(a) What do you think metal M is?
(b) What could be gas X2?
(c) Name the metal salt MX.
(d) Name any two salt solutions which on mixing together can produce a precipitate of salt MX.
(e) What type of chemical reaction takes place when salt MX is exposed to light? Write the equation of the reaction?
How will you obtain Nitrogen dioxide from lead nitrate.
Also give balanced equations for the reactions
Give a balanced equation for –
A thermal decomposition reaction involving heat on limestone [calcium carbonate]
Complete the statement by filling in the blank with the correct word:
Decomposition of silver salts in the presence of sunlight is an example of _________.
Which one of the following processes involves chemical reactions?
Which of the following is an endothermic process?

- Identify the gasses evolved at the anode and cathode in the above experimental set up.
- Name the process that occurs. Why is it called so?
- Illustrate the reaction of the process with the help of a chemical equation.
