Advertisements
Advertisements
प्रश्न
What is the corrosion of iron known as?
उत्तर
The corrosion of iron is known as rusting. Iron is a reactive metal and corrodes (rusts) in the presence of air (oxygen) and water (dampness).
APPEARS IN
संबंधित प्रश्न
Write a chemical equation to show the process of corrosion of iron.
Explain why, iron sheets are coated with zinc.
Fill in the following blank with suitable word:
The corrosion of copper produces a .............. coating of basic copper carbonate on its surface
What are the constituents of stainless steel?
Compare roasting and calcination.
No chemical reaction takes place when granules of a solid, A, are mixed with the powder of another solid, B. However when the mixture is heated, a reaction takes place between its components. One of the products, C, is a metal and settles down in the molten state while the other product, D, floats over it. It was observed that the reaction is highly exothermic.
(i) Based on the given information make an assumption about A and B and write a chemical equation for the chemical reaction indicating the conditions of reaction, physical state of reactants and products and thermal status of reaction.
(ii) Mention any two types of reactions under which above chemical reaction can be classified.
Write scientific reasons.
Lemon or tamarind is used for cleaning copper vessels turned greenish.
Complete the process of iron rusting by filling the blanks. Suggest a way to prohibit the process.
The iron rust is formed due to........................... reaction. Different
regions on iron surface become anode and cathode.
Reaction on anode region :
`F_e(s) → Fe^(2+) (aq) +2e^-`
Reaction on anode region :
`O_2(g) + 4H^+(aq) +............................ → 2H_2 O (l) `
When Fe2+ ions migrate from anode region they react with ................... to form Fe3+ ions.
A reddish coloured hydrated oxide is formed from ............... ions. It is called rust.
`2Fe_(3+) (aq) + 4H_2O(l) → ................. + 6H_+(aq) `
A way to prevent rusting ..................................................................
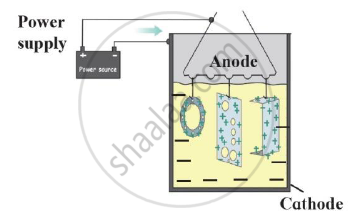
Identify the process shown in the diagram and explain it in short
Explain the term – rusting and give a word equation for the formation of rust. If polished iron nails are kept in three separate test tubes, state the contents in each test tube required, to prove the conditions for rusting.
State whether the statement given below is true or false. If false write the correct statement.
Either oxygen or moisture is essential for rusting.
Find the odd one out and give its explanation.
Match the columns.
| Group A | Group B |
| 1) Electroplating | a) Pressure cooker |
| 2) Anodising | b) Silver plated spoons |
| c) Coating of tin on copper | |
| d) Coating of Zinc on iron |
Write the name.
An alloy of copper and tin-
Write the name.
Method used to prevent corrosion of copper.
Write scientific reason.
Coins are made from metals and alloys.
Observe the following diagram and give answers.
- Name this method of prevention of corrosion.
- For prevention of which metal this method is used?
- What is used as anode in this method?
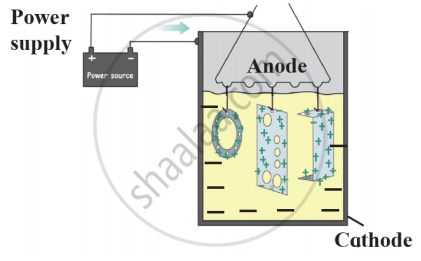
Observe the following figure and write the answer of the question.

- Which process is shown in the figure?
- Explain the chemical reaction shown in the figure.
- Write the reactions on anode and cathode.
A man painted his main gate made up of iron, to
- prevent it from rusting.
- protect it from the sun.
- make it look beautiful.
- make it dust-free.
Which of the above statement(s) is/are correct?
Explain the chemical reactions in rusting of iron.
