Advertisements
Advertisements
प्रश्न
What happens when (CH3)3 C – OH is heated with Cu/573 K?
Write the chemical equation in support of your answer.
उत्तर
When a tertiary alcohol is heated with Copper at 573 K, due to absence of α-hydrogen atom dehydration reaction takes place and produce 2-methyl propene.
\[\begin{array}{cc}
\phantom{....}\ce{CH3}\phantom{.................}\ce{CH3}\phantom{.}\\
\phantom{.}|\phantom{.....................}|\phantom{.}\\
\phantom{}\ce{CH3 - C - OH ->[Cu][573K] \underset{2-metyl propene}{CH3 - C = CH2}}\\
|\phantom{......................}\\
\ce{\underset{Tertiary Butanol}{CH3}}\phantom{...................}
\end{array}\]
APPEARS IN
संबंधित प्रश्न
Write the mechanism of the following reaction :
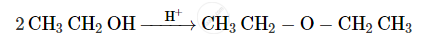
Write the mechanism of the following reaction :
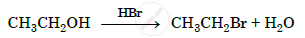
Lucas reagent is ____________.
Which of the following are used to convert RCHO into RCH2OH?
(i) H2/Pd
(ii) LiAlH4
(iii) NaBH4
(iv) Reaction with RMgX followed by hydrolysis
Which one of the following on oxidation gives a ketone?
Primary and secondary alcohols on the action of reduced copper give:
What is Lucas reagent?
Which of the following observation is shown by 2-phenyl ethanol with Lucas Reagent?
Write the mechanism of acid dehydration of ethanol to yield ethene.
Write the mechanism of acid-catalysed dehydration of ethanol to yield ethene.
