Advertisements
Advertisements
प्रश्न
What information do the following chemical equations convey? Mg + 2HCl → MgCl2+ H2
उत्तर
This equation conveys the following information:
Magnesium reacts with hydrochloric acid to form magnesium chloride and hydrogen gas.
24 g of magnesium reacts with 2(1 + 35.5) = 73 g of hydrochloric acid to produce (24 + 71), i.e. 95 g of magnesium chloride.
Hydrogen produced at STP is 22.4 litres.
APPEARS IN
संबंधित प्रश्न
With the help of an appropriate example, justify that some of the chemical reactions are determined by Change in colour
Give chemical equation for the reaction involved in the above case.
Balance the following chemical equation:
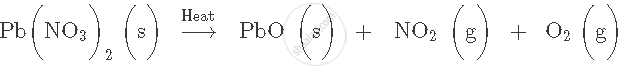
Balance the following equation. Also name the product formed.
`"NaNO"_3 → "NaNO"_2 + "O"_2` Name of product (s)
Write down what you understood from the following chemical reaction.
AgNO3(aq) + NaCI(aq) → AgCI ↓ + NaNO3(aq)
\[\ce{MnO2 + 4HCl -> MnCl2 + 2H2O + Cl2}\]
0.02 moles of pure MnO2 is heated strongly with conc. HCl. Calculate the mass of chlorine gas formed.
A chemical reaction is generally accompanied by certain external indications or characteristics. These include – change of – (a) colour (b) state (c) smell (d) evolution of gas (e) formation of precipitate (f) evolution or absorption of heat. With reference to change of colour – state the change in colour seen when the following are heated – copper carbonate.
Name the following:
Two gases which react under pressure in presence of a catalyst at elevated temperatures to give a gaseous product.
Give an example of a chemical equation in which two reactants form:
three product
Balance the following simple equation:
Al + N2 → AlN
Balance the following simple equation:
K + CO2 → K2O + C
