Advertisements
Advertisements
प्रश्न
Which of the following compounds will react with sodium hydroxide solution in water?
विकल्प
\[\ce{C6H5OH}\]
\[\ce{C6H5CH2OH}\]
\[\ce{(CH3)3COH}\]
\[\ce{C2H5OH}\]
उत्तर
\[\ce{C6H5OH}\]
Explanation:
Phenol being more acidic reacts with sodium hydroxide solution in water to give sodium phenoxide which is resonance stabilized.
Alcohols are very weak acids.
\[\ce{C6H5OH + NaOH -> C6H5ONa + H2O}\]
APPEARS IN
संबंधित प्रश्न
Write IUPAC name of the following compound:
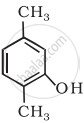
Write the IUPAC name of the following compound:
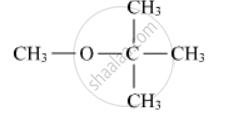
Write the IUPAC name of the following :
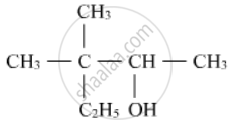
Write IUPAC name of the following
\[\begin{array}{cc}\ce{CH3-CH-CH-CH2-OH}\\|\phantom{.....}|\phantom{.......}\\\ce{OH}\phantom{..}\ce{CH3}\phantom{.....}\end{array}\]
The major product formed by the reaction:
\[\begin{array}{cc}
\ce{CH3CH-CH2Br ->[CH3O^-][CH3OH] is}\\
|\phantom{................}\\
\ce{CH3}\phantom{.............}
\end{array}\]
The heating of phenyl methyl ether with HI produces:
Explain why p-nitrophenol is more acidic than phenol.
Assertion: Like bromination of benzene, bromination of phenol is also carried out in the presence of Lewis acid.
Reason: Lewis acid polarises the bromine molecule.
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{................}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{..}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{................}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{...}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
