Advertisements
Advertisements
प्रश्न
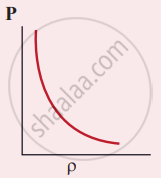
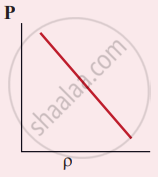
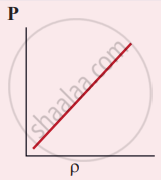
Which of the following shows the correct relationship between the pressure and density of an ideal gas at constant temperature?
विकल्प
उत्तर
APPEARS IN
संबंधित प्रश्न
Two identically sized rooms A and B are connected by an open door. If the room A is air-conditioned such that its temperature is 4°C lesser than room B, which room has more air in it?
The ratio γ = `"C"_"p"/"C"_"v"` for a gas mixture consisting of 8 g of helium and 16 g of oxygen is ____________.
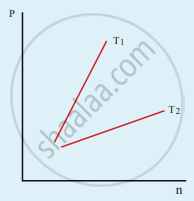
The following graph represents the pressure versus number density for an ideal gas at two different temperatures T1 and T2. The graph implies
What is the microscopic origin of pressure?
A gas is at temperature 80°C and pressure 5 × 10−10 Nm−2. What is the number of molecules per m3 if Boltzmann’s constant is 1.38 × 10−23 J K−1
If 1020 oxygen molecules per second strike 4 cm2 of wall at an angle of 30° with the normal when moving at a speed of 2 × 103 ms−1, find the pressure exerted on the wall. (mass of one oxygen atom = 2.67 × 10−26 kg)
Estimate the total number of air molecules in a room of a capacity of 25 m3 at a temperature of 27°C.
What is an ideal gas?
The kinetic energy per molecule of a gas at temperature T is ______.
The velocities of five molecules are 2 m/s, 3 m/s, 4 m/s, 5 m/s and 6 m/s. Find the root mean square velocity of molecules.