Advertisements
Advertisements
प्रश्न
Which of the following statements are correct?
(i) S – S bond is present in \[\ce{H2S2O6}\].
(ii) In peroxosulphuric acid \[\ce{(H2SO5)}\] sulphur is in +6 oxidation state.
(iii) Iron powder along with \[\ce{Al2O3}\] and \[\ce{K2O}\] is used as a catalyst in the preparation of \[\ce{NH3}\] by Haber’s process.
(iv) Change in enthalpy is positive for the preparation of \[\ce{SO3}\] by catalytic oxidation of \[\ce{SO2}\].
उत्तर
(i) S – S bond is present in \[\ce{H2S2O6}\].
(ii) In peroxosulphuric acid \[\ce{(H2SO5)}\] sulphur is in +6 oxidation state.
Explanation:
\[\begin{array}{cc}
\ce{O}\phantom{...}\ce{O}\\
||\phantom{...}||\\
\underset{//\phantom{...}|\phantom{........}|\phantom{...}\backslash\backslash}{\ce{S}-\ce{S}}\\
\ce{O}\phantom{..}\ce{OH}\phantom{.}\ce{OH}\phantom{.}\ce{O}
\end{array}\]
In \[\ce{H2SO5}\], there is a peroxo-linkage.
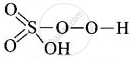
(O in peroxide linkage has oxidation state – 1)
APPEARS IN
संबंधित प्रश्न
Write the conditions to maximize the yield of H2SO4 by contact process.
Complete the following equations: C + conc. H2SO4 →
Describe the manufacture of H2SO4 by contact process?
What happens when dilute sulphuric acid is treated with Fe
Write chemical reactions for different steps in the manufacture of sulphuric acid by lead chamber process. Draw the structure of phosphorous pentachloride
Write the reaction of conc. \[\ce{H2SO4}\] with sugar.
Which of the following reaction is NOT involved in contact process used for manufacturing sulfuric acid?
Gas 'X' is prepared by treating sodium sulfite with dilute sulfuric acid. When gas 'X' is oxidised by dioxygen in presence of vanadium (V) oxide, gas 'Y' is formed. Identify X and Y.
The molecular formula of oleum is ____________.
Hot conc. \[\ce{H2SO4}\] acts as the moderately strong oxidising agent. It oxidises both metals and non-metals. Which of the following element is oxidised by conc. \[\ce{H2SO4}\] into two gaseous products?
