Advertisements
Advertisements
प्रश्न
Which of the following statements is wrong about alkanes?
विकल्प
They are all saturated hydrocarbons.
They can undergo addition as well as substitution reaction.
They are almost non-polar in nature.
On complete combustion, they give out carbon dioxide and water.
उत्तर
They can undergo addition as well as substitution reaction.
Explanation:
Alkanes only undergo substitution reactions (like halogenation). Addition reactions are shown by unsaturated hydrocarbons like alkenes and alkynes.
APPEARS IN
संबंधित प्रश्न
The compound formed where two alkyl groups are linked by 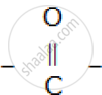 group.
group.
Using their structural formulae identify the functional group by circling them
Dimethyl ether
Name the products formed and write an equation when ethyne is added to the bromine in an
inert solvent ?
Name the products formed and write an equation when ethyne is added to the iodine in an
inert solvent ?
Name the hydrocarbon which is a linear molecule.
What is the special feature of the structure of ethyne?
Choose the correct answer from the options given below :
If the molecular formula of an organic compouns is C10H18 it is
State how the following conversion can be carried out:
Ethyl chloride to Ethyl alcohol
Convert ethyne to ethane.
Alkynes undergo ______ reactions.
