Advertisements
Advertisements
प्रश्न
Write a balanced chemical reaction of sulfuric acid with carbon.
उत्तर
\[\ce{\underset{\text{Carbon}}{C} + \underset{\text{Sulfuric acid (Conc.)}}{2H2SO4} -> \underset{\text{Carbon dioxide}}{CO2} + \underset{\text{Sulfur dioxide}}{2SO2} + 2H2O}\]
APPEARS IN
संबंधित प्रश्न
Answer the following.
What is the oxidation state of ‘S’ in H2SO4?
Write the names and structural formulae of oxoacids of chlorine.
Answer the following.
What happens when Br2 reacts with excess of F2.
Write a balanced chemical reaction of sulfuric acid with sulfur.
What is oxidation state of sulfur in the following?
Sulfuric acid
What is oxidation state of sulfur in the following?
Peroxymonosulfuric acid
Write three physical properties of sulfuric acid.
Write three uses of sulfuric acid.
The number of S - OH bond in thiosulfuric acid is ____________.
How many numbers of P—O—H and P—O—P bonds are present in pyrophosphoric acid respectively?
The following structure represents
In which of the following oxoacids 'Cl' exhibit +5 oxidation state?
The molecular formula of peroxymonosulfuric acid is ____________.
Which of the following structure represents thiosulfuric acid?
Which of the following oxyacid of sulphur contain S-O-S linkage?
Which of the following halogen does NOT form perhalic acid?
Which of the following oxyacid of sulphur contain both S=S and S=O bonds?
How many lone pair of electrons are present on chlorine atoms in Hypochlorous acid?
Write the structure of HClO4.
Which among the following oxoacids of phosphorus shows a tendency of disproportionation?
Draw the structure of oxyacid of sulphur in which the oxidation state of sulphur is + 4.
How is chlorine obtained by oxidation of HCl. Write the reactions using two oxidizing agents.
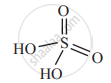
Draw structure of H2SO4.
Draw the structure of hypochlorous acid.
