Advertisements
Advertisements
प्रश्न
Write the structures of A, B, C, D and E in the following reactions:
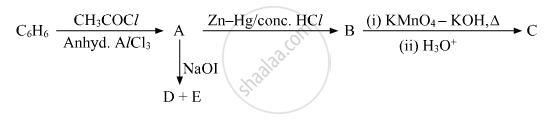
उत्तर

APPEARS IN
संबंधित प्रश्न
Give simple chemical tests to distinguish between the following pairs of compounds: Benzoic acid and Phenol
Write the main products when
2, 4, 6-trinitrochlorobenzene is subjected to hydrolysis
Name the reagent used in the following reaction:
Bromination of phenol to 2, 4, 6-tribromophenol.
When phenol is heated with CHCl3 and alcoholic KOH when salicylaldehyde is produced. This reaction is known as ____________.
The electrophile involved in Reimer-Tiemann reaction of phenol with CHCl3 in presence of NaOH:
Which of the following species can act as the strongest base?
Phenol does not undergo nucleophilic substitution reaction easily due to ______.
Which of the following are benzylic alcohols?
(i) \[\ce{C6H5 - CH2 - CH2OH}\]
(ii) \[\ce{C6H5 - CH2OH}\]
(iii) \[\begin{array}{cc}
\ce{C6H5 - CH - OH}\\
\phantom{}|\phantom{.}\\
\phantom{..}\ce{CH3}\phantom{}
\end{array}\]
(iv) \[\begin{array}{cc}
\ce{C6H5 - CH2 - CH - OH}\\
\phantom{.......}|\phantom{}\\
\phantom{.........}\ce{CH3}\phantom{}
\end{array}\]
Why ortho-nitrophenol is steam volatile while para-nitrophenol is not?
