Advertisements
Advertisements
प्रश्न
Write the answer to the questions with reference to the periodic table.

- What do (A), (B), (C), and (D) represent? [1]
- Elements are arranged in increasing order of their ______. [1]
- Write the electronic configuration of the first four elements in Group I. [1]
- What change in atomic radius is observed in moving from left to right in a Period? [1]
- Give a reason for (d). [1]
उत्तर
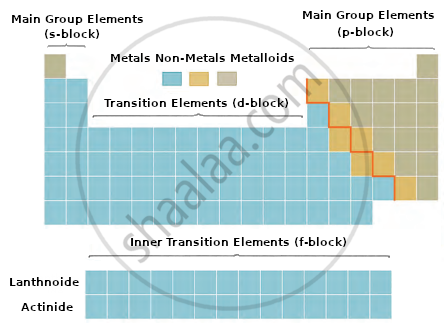
(A) represents s-block elements.
(B) represents p-block elements.
(C) represents transition elements (d-block).
(D) represents inner transition elements, i.e., (f-block).- Elements are arranged in increasing order of their atomic numbers.
- The electronic configuration for elements is as follows:
Elements Electronic configuration H 1 Li 2, 1 Na 2, 8, 1 K 2, 8, 8, 1 - Atomic radius goes on decreasing while going from left to right within a period.
-
The positive charge on the nucleus grows by one unit at a time as the atomic number rises sequentially while moving from left to right over time. However, the same outermost shell receives the additional electron as well. The electrons are more firmly drawn into the nucleus due to the higher nuclear charge, which reduces the size of the atom.
APPEARS IN
संबंधित प्रश्न
Write the name from the description.
The period with electrons in the shells K, L, and M.
Write the name from the description.
The family of metals having valency one.
Write the name from the description.
The family of metals having valency two.
Write the name from the description.
Nonmetals in the third period.
Write the name from the description.
Two elements having valency 4.
Write a short note on the following type of element –
inner transition elements
Write a short note on the following type of element –
normal elements
Name or state following with reference to the element of the first three periods of the periodic table.
The periods which contain the inner transition elements.
d-block elements are otherwise known as ______.
f-block elements are also known as ______.
______ block element is placed at the bottom of the periodic table.
What are the s-block elements?
d-block elements are called transition elements.
Match the columns:
| Column ‘A’ | Column ‘B’ |
| Manganese | (a) Metal |
| (b) Non-metal | |
| (c) Transition metal |
Answer the following questions related to the modern periodic table.
Which block elements are known as transition elements?
Answer the following questions related to the modern periodic table.
How are elements placed around the zig-zag line in the p-block of the periodic table?
