Advertisements
Advertisements
प्रश्न
Write the chemical formula of the following compound in a step-by-step manner –
Magnesium nitride
उत्तर
Write the formula of
| Magnesium nitride | Symbol | Valency |
| Magnesium | \[\ce{Mg}\] | \[\ce{2^{+}}\] |
| Nitride | \[\ce{N}\] | \[\ce{3^{-}}\] |
Step I – Write each symbol with its valency.
Positive ion is written first
\[\ce{Mg}^{2+}\] \[\ce{N}^{3-}\]
Step II – Interchange the valencies positive ion is written first.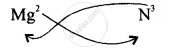
The Formula is \[\ce{Mg3N2}\]
APPEARS IN
संबंधित प्रश्न
Write the molecular formulae for the following compounds and name the elements present of Common salt.
Write the molecular formulae for the following compounds and name the elements present of Nitric acid.
The valency of aluminium is 3. Write the valency of other radicals present in the following compounds of Aluminium oxide.
Write the molecular formulae of Calcium hydroxide.
Copper having electronic configuration 2,8,18,1 exhibits variable valency. Give a reason for the same and name the compound CuCI and CuCl2.
Write the chemical formula of the following compound in a step-by-step manner –
Sodium bromide
Write the chemical formula of the following compound in a step-by-step manner –
Calcium hydroxide
Write the chemical formula of the following compound in a step-by-step manner –
Zinc hydroxide
Write the chemical formula of the following compound in a step-by-step manner –
Iron [II] hydroxide
Match the compounds in List I – 1 to 20 with their correct formulas in List II – A to T.
| List I | List II |
| 1. Copper [I] sulphide | A: KMnO4 |
| 2. Potassium permanganate | B: Mg3N2 |
| 3. Phosphoric acid | C: Mg(NO3)2 |
| 4. Copper [I] oxide | D: Al2(SO4)3 |
| 5. Carbonic acid | E: Na2ZnO2 |
| 6. Aluminium sulphide | F: N2O |
| 7. Iron [II] oxide | G: H2CO3 |
| 8. Iron [III] sulphide | H: Al2S3 |
| 9. Iron [II] sulphate | I: NO |
| 10. Sodium zincate | J: FeS |
| 11. Nitrous oxide | K: Fe2S3 |
| 12. Aluminium sulphate | L: H3PO4 |
| 13. Magnesium nitride | M: Cu2S |
| 14. Iron [III] sulphate | N: CuS |
| 15. Copper [II] oxide | O: Fe2O3 |
| 16. Iron [III] oxide | P: FeO |
| 17. Nitric oxide | Q: FeSO4 |
| 18. Copper [II] sulphide | R: Fe2(SO4)3 |
| 19. Iron [II] sulphide | S: CuO |
| 20. Magnesium nitrate | T: Cu2O |
