Advertisements
Advertisements
प्रश्न
Write the preparation reactions for acid amide from the following.
Carboxylic acid
उत्तर
Carboxylic acids react with ammonia to form ammonium carboxylate salt which on further strong heating at high temperature decomposes to give acid amide.
\[\begin{array}{cc}
\phantom{}\ce{O}\phantom{......==.............}\ce{O}\phantom{........................}\ce{O}\phantom{...}\\
\phantom{}||\phantom{.......................}||\phantom{.........................}||\phantom{...}\\
\ce{\underset{\text{Carboxylic acid}}{R - C - OH} + NH3 ⇌ \underset{\text{Ammonium carboxylate}}{R - C - O- - NH^+_4} ->[\Delta][-H2O] \underset{\text{Acid amide}}{R - C - NH2}}
\end{array}\]
APPEARS IN
संबंधित प्रश्न
Arrange the following carboxylic acids with increasing order of their acidic strength and justify your answer.
1) ![]()
2) ![]()
3) ![]()
Correct order of acid strength for
- acetic acid
- fluoroacetic acid
- 4-Nitrobenzoic acid
- 4-Methyl benzoic acid is ______.
Write the structure of the product formed when carboxylic acid is heated with a dehydrating agent like P2O5
Write the reducing agent which CANNOT reduce –COOH group.
Write reactions for the following conversions.
Propanone to Propane
Write reactions for the following conversions.
4-Nitrobenzoic acid to Nitrobenzene
Write chemical reactions to convert –COOH group of acetic acid into the following.
CH3COCl
Explain the acidic nature of carboxylic acids.
Write reactions for the action of the following reagents on p-chlorobenzaldehyde.
Ethane-1,2-diol in presence of dry HCl.
Complete the following sequence of reactions and write structures for A, B, C.
\[\ce{Dry ice ->[i. Dry ether][ii. Hydrolysis] A ->[PCl5] B ->[H2(gas)][Pd - BaSO4] C}\]
In the reaction,
\[\ce{CH3COOH ->[SOCl2] X ->[Sodium salt of carboxylic acid] Y}\].
The compound Y was found to be a mixed acid anhydride. Thus, the sodium salt of carboxylic acid used CANNOT be ____________.
In the reaction, \[\ce{C6H5COCH3 ->[{[H]}][Zn-Hg/conc. HCl] X}\], X is ______.
Which is the gas evolved when carboxylic acids react with strongly electropositive metals (such as Na, K, Ca, Zn)?
Which of following elements does not form amide when reacted with ammonia?
In the following reaction:
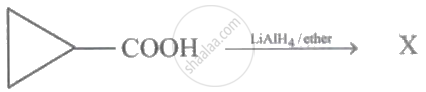
The product X is:
A flavouring agent found in oil of wintergreen is ______.
Which of the following carboxylic acids will have the highest acidity?
The elimination of CO2 from a carboxylic acid is known as ____________.
Which of the following compounds is obtained when ethanoic anhydride is treated with water?
Excess of ammonia with sodium hypochloride solution in the presence of glue or gelatine gives ______.
Which of the following can reduce?
\[\ce{RCOOH -> RCH2OH}\]
How sodium bicarbonate test is used to distinguish between carboxylic acid and phenol?
How will you prepare Acetic anhydride from acetic acid?
