Advertisements
Advertisements
प्रश्न
Write the preparation reactions for acid amide from the following.
Carboxylic acid
उत्तर
Carboxylic acids react with ammonia to form ammonium carboxylate salt which on further strong heating at high temperature decomposes to give acid amide.
\[\begin{array}{cc}
\phantom{}\ce{O}\phantom{......==.............}\ce{O}\phantom{........................}\ce{O}\phantom{...}\\
\phantom{}||\phantom{.......................}||\phantom{.........................}||\phantom{...}\\
\ce{\underset{\text{Carboxylic acid}}{R - C - OH} + NH3 ⇌ \underset{\text{Ammonium carboxylate}}{R - C - O- - NH^+_4} ->[\Delta][-H2O] \underset{\text{Acid amide}}{R - C - NH2}}
\end{array}\]
APPEARS IN
संबंधित प्रश्न
Answer in brief.
Formic acid is stronger than acetic acid. Explain.
Correct order of acid strength for
- acetic acid
- fluoroacetic acid
- 4-Nitrobenzoic acid
- 4-Methyl benzoic acid is ______.
Write the structure of the product formed when carboxylic acid is heated with a dehydrating agent like P2O5
What is the action of following on proponal?
Hydroxyl amine
Write reactions for the following conversions.
Propanone to Propane
Write chemical reactions to convert –COOH group of acetic acid into the following.
CH4
Write chemical reactions to convert –COOH group of acetic acid into the following.
CH3COCl
Write reaction for preparation of acetophenone from benzoyl chloride.
Write reactions for the action of the following reagents on p-chlorobenzaldehyde.
Ethane-1,2-diol in presence of dry HCl.
In the reaction,
\[\ce{CH3COOH ->[SOCl2] X ->[Sodium salt of carboxylic acid] Y}\].
The compound Y was found to be a mixed acid anhydride. Thus, the sodium salt of carboxylic acid used CANNOT be ____________.
In the reaction, \[\ce{C6H5COCH3 ->[{[H]}][Zn-Hg/conc. HCl] X}\], X is ______.
Which is the gas evolved when carboxylic acids react with strongly electropositive metals (such as Na, K, Ca, Zn)?
The product of the reaction between dialkyl cadmium and acyl chloride is a/an ____________.
Which of following elements does not form amide when reacted with ammonia?
In the following reaction:
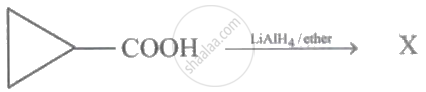
The product X is:
Which of the following carboxylic acids will have the highest acidity?
The elimination of CO2 from a carboxylic acid is known as ____________.
What amount of dinitrogen contains 3.6 × 1018 molecules?
The reducing agent preferred to convert carboxylic acids and esters to primary alcohols is ____________.
Write structure of adipic acid.
Excess of ammonia with sodium hypochloride solution in the presence of glue or gelatine gives ______.
Which of the following can reduce?
\[\ce{RCOOH -> RCH2OH}\]
Draw structures 01 conjugate bases of monochloroacetic acid and dichloroacetic acid. Which one is more stabilized by -I effect?
