Advertisements
Advertisements
प्रश्न
Write the structures of two compounds having molecular formula C3H6O and give their names.
उत्तर
The isomers of C3H6O include:
propanal
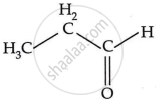
propanone
\[\begin{array}{cc}
\ce{H}\phantom{...}\ce{O}\phantom{...}\ce{H}\\
|\phantom{....}||\phantom{....}|\\
\ce{H - C - C - C - H}\\
|\phantom{.........}|\\
\ce{H}\phantom{........}\ce{H}
\end{array}\]
APPEARS IN
संबंधित प्रश्न
Give reason why the carbon compounds generally have low melting and boiling points.
Fill in the following blank with suitable word:
CnH2n is the general formula of .......... hydrocarbons.
What is the unique property of carbon atom? How is this property helpful to us?
What are hydrocarbons? Explain with examples.
The solid element A exhibits the property of catenation. It is also present in the form of a gas B in the air which is utilised by plants in photosynthesis. An allotrope C of this element is used in glass cutters.
(a) What is element A?
(b) What is the gas B?
(c) Name the allotrope C.
(d) State another use of allotrope C (other than in glass cutters).
(e) Name another allotrope of element A which exists as spherical molecules.
(f) Name a yet another allotrope of element A which conduct electricity.
You are given the following molecular formulae of some hydrocarbons:
C5H8; C7H14; C6H6; C5H10; C7H12; C6H12
Which three formulae represent open chain unsaturated hydrocarbons having double bonds?
Pentane has the molecular formula C5H12. It has ______.
Catenation is the ability of an atom to form bonds with other atoms of the same element. It is exhibited by both carbon and silicon. Compare the ability of catenation of the two elements. Give reasons.
How will you prove that C4H8 and C5H10 are homologues?
Recognise the carbon chain type of the following:
\[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\phantom{....}|\phantom{....}|\\\ce{H - C - C - C - C - H}\\|\phantom{....}|\phantom{....}|\phantom{....}|\\
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}
\end{array}\]
