Advertisements
Advertisements
Question
Write the structures of two compounds having molecular formula C3H6O and give their names.
Solution
The isomers of C3H6O include:
propanal
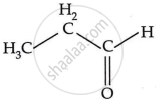
propanone
\[\begin{array}{cc}
\ce{H}\phantom{...}\ce{O}\phantom{...}\ce{H}\\
|\phantom{....}||\phantom{....}|\\
\ce{H - C - C - C - H}\\
|\phantom{.........}|\\
\ce{H}\phantom{........}\ce{H}
\end{array}\]
APPEARS IN
RELATED QUESTIONS
List two reasons for carbon forming a large number of compounds. Name the type of bonding found in most of its compounds. Why does carbon form compounds mainly by this kind of bonding?
Draw the structures for Ethanoic acid.
Draw the structures of possible isomers of butane, C4H10.
What is the unique property of carbon atom? How is this property helpful to us?
Giving their structures, state the number of single bonds, double bonds and triple bonds (if any) in the following compounds:
benzene
The hydrocarbon which has alternate single and double bonds arranged in the form of a ring is:
(a) cyclobutane
(b) benzene
(c) butene
(d) hexene
Two organic compounds A and B have the same molecular formula C6H12. Write the names and structural formulae:
Which compound contains single bonds as well as a double bond?
Pentane has the molecular formula C5H12. It has ______.
How many electrons are there in the outermost orbit of carbon?
Recognise the carbon chain type of the following:
\[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\phantom{....}|\phantom{....}|\\\ce{H - C - C - C - C - H}\\|\phantom{....}|\phantom{....}|\phantom{....}|\\
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}
\end{array}\]
