English Medium
Academic Year: 2022-2023
Date & Time: 4th March 2023, 10:30 am
Duration: 3h
Advertisements
General Instructions:
- This question paper consists of 39 questions in 5 sections.
- All questions are compulsory. However, an internal choice is provided in some questions. Students are expected to attempt only one of these questions.
- Section A consists of 20 objective type questions carrying 1 mark each. Q. No. 17 to 20 are Assertion - Reasoning based questions.
- Section B consists of 6 Very Short questions carrying 02 marks each. Answers to these questions should be in the range of 30 to 50 words.
- Section C consists of 7 Short Answer type questions carrying 03 marks each. Answers to these questions should be in the range of 50 to 80 words
- Section D consists of 3 Long Answer type questions carrying 05 marks each. Answers to these questions should be in the range of 80 to 120 words.
- Section E consists of 3 source-based/case-based units of assessment of 04 marks each with sub-parts.
- There is no overall choice. However, an internal choice has been provided in some Sections.
To balance the following chemical equation the value of x and y should respectively be:
\[\ce{2NaOH + xAl_2O_3->yNaAlO_2 + H_2O}\]
1, 4
1, 2
2, 4
2, 3
Chapter: [0.01] Chemical Reactions and Equations
A solution turns the colour of turmeric to reddish brown. If the same solution is poured on universal indictor, its colour would change to ______.
violet
blue
red
green
Chapter: [0.02] Acids, Bases and Salts
Select the appropriate state symbols of the products given as X and Y in the following chemical equation by choosing the correct option from table given below:
\[\ce{Zn_{(s)} + H_2SO_{4(l)} ->ZnSO_{4(X)} + H_{2(Y)}}\]
(X) - (s); (Y) - (l)
(X) - (aq); (Y) - (g)
(X) - (aq); (Y) - (s)
(X) - (g); (Y) - (aq)
Chapter: [0.03] Metals and Non Metals
Two salts 'X' and 'Y' are dissolved in water separately. When phenolphthalein is added to these two solutions, the solution 'X' turns pink and the solution 'Y' does not show any change in colour, therefore 'X' and 'Y' are:
(X) - \[\ce{Na_2CO_3}\]; (Y) - \[\ce{NH_4Cl}\]
(X) - \[\ce{Na_2SO_4}\]; (Y) - \[\ce{NaHCO_3}\]
(X) - \[\ce{NH_4Cl}\] ; (Y) - \[\ce{Na_2SO_4}\]
(x) - \[\ce{NaNO_3}\] ; (Y) - \[\ce{Na_2SO_4}\]
Chapter: [0.02] Acids, Bases and Salts
Given below are two columns, Column I shows enzymes secreted by the glands in the alimentary canal of human beings and Column II indicates the components of food on which enzymes act. Choose the options showing correct matching:
|
Column I |
Column II (Component) |
| Pepsin | Starch |
| Column I (Enzymes) |
Column II (Component) |
| Trypsin | Proteins |
| Column I (Enzymes) |
Column II (Component) |
| Lipase | Proteins |
| Column I (Enzymes) |
Column II (Component) |
| Amylase | Emulsified fat |
Chapter: [0.05] Life Processes
Walking in a straight line and riding a bicycle are the activities which are possible due to a part of the brain. Choose the correct location and name of this part from the given option :
| Part of the brain | Name |
| Fore brain | Cerebrum |
| Part of the brain | Name |
| Mid brain | Hypothalamus |
| Part of the brain | Name |
| Hind brain | Cerebellum |
| Part of the brain | Name |
| Hind brain | Medulla |
Chapter: [0.06] Control and Co-ordination [0.06] Control and Co-ordination
To obtain a magnification of +2 with a concave mirror of radius of curvature 60 cm the object distance must be ______.
-90 cm
-45 cm
-30 cm
-15 cm
Chapter: [0.09] Light - Reflection and Refraction
Bronze is an alloy of ______.
copper and tin
copper and zinc
tin and zinc
copper, zinc and tin
Chapter: [0.01] Chemical Reactions and Equations [0.03] Metals and Non Metals
In peas, a pure tall plant (TT) is crossed with a pure short plant (tt). The ratio of pure tall plants to pure short plants in F2 generation will be ______.
1 : 3
3 : 1
1 : 1
2 : 1
Chapter: [0.08] Heredity
Study the given figure of a Food web and identify the primary consumer in the food web:

Mice and Bear
Rabbit and Cat
Rabbit and Fox
Mice and Rabbit
Chapter: [0.13] Our Environment
Choose the correct order of the stages of binary fission in Leishmania.
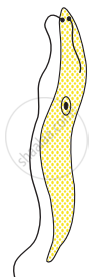 |
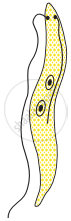 |
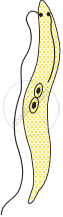 |
 |
 |
| (I) | (II) | (III) | (IV) | (V) |
I, II, III, IV, V
I, III, II, V, IV
I, III, V, II, IV
I, II, III, V, IV
Chapter: [0.07] How do Organisms Reproduce?
Consider the following chemical equation I and II
- \[\ce{Mg + 2HCl -> MgCl_2 +H_2}\]
- \[\ce{NaOH +HCl -> NaCl + H_2O}\]
The correct statement about these equations is:
'I' is a displacement reaction and 'II' is a decomposition reaction.
'I' is a displacement reaction and 'II' is double displacement reaction.
Both 'I' and 'II' are displacement reactions.
Both 'I' and 'II' are double- displacement reactions.
Chapter: [0.01] Chemical Reactions and Equations
The phenomena of light involved in the formation of rainbow are ______.
Reflection, refraction and dispersion
Refraction, dispersion and total internal reflection
Refraction, dispersion and internal reflection
Dispersion, scattering and total internal reflection
Chapter: [0.1] The Human Eye and the Colourful World
Consider the following three flowers namely X, Y and Z. Which flower(s) would develop into a fruit?
| Flower X | Flower Y | Flower Z |
 |
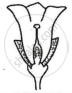 |
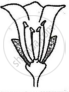 |
'X' only
'Z' only
'X' and 'Y' only
'Y' and 'Z'
Chapter: [0.07] How do Organisms Reproduce?
The magnetic field inside a long straight solenoid carrying current ______.
is zero
decreases as we move towards its end.
increases as we move towards its end.
is same at all points.
Chapter: [0.12] Magnetic Effects of Electric Current
In human eye the part which allows light to enter into the eye is ______.
Retina
Pupil
Eye lens
Cornea
Chapter: [0.1] The Human Eye and the Colourful World
Assertion (A): It is advised that while diluting an acid one should add water to acid and not acid to water keeping the solution continuously stirred.
Reason (R): The process of dissolving an acid into water is highly exothermic.
Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of (A).
Both Assertion (A) and Reason (R) are true but Reason (R) is not the correct explanation of (A).
Assertion (A) is true, but Reason (R) is false.
Assertion (A) is false, but Reason (R) is true.
Chapter: [0.02] Acids, Bases and Salts
Assertion (A): An ecosystem consists of biotic components and abiotic components.
Reason (R): Biotic and abiotic components play important roles for the sustenance of life and work independently in all ecosystems.
Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of (A).
Both Assertion (A) and Reason (R) are true but Reason (R) is not the correct explanation of (A).
Assertion (A) is true, but Reason (R) is false.
Assertion (A) is false, but Reason (R) is true.
Chapter: [0.13] Our Environment
Assertion (A): Amoeba takes in food using finger like extensions of the cell surface.
Reason (R): In all unicellular organisms, the food is taken in by the entire cell surface.
Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of (A).
Both Assertion (A) and Reason (R) are true but Reason (R) is not the correct explanation of (A).
Assertion (A) is true, but Reason (R) is false.
Assertion (A) is false, but Reason (R) is true.
Chapter: [0.05] Life Processes
Assertion (A): Melting point and boiling point of ethanol are lower than that of sodium chloride.
Reason (R): The forces of attraction between the molecules of ionic compounds are very strong.
Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of (A).
Both Assertion (A) and Reason (R) are true but Reason (R) is not the correct explanation of (A).
Assertion (A) is true, but Reason (R) is false.
Assertion (A) is false, but Reason (R) is true.
Chapter: [0.03] Metals and Non Metals [0.04] Carbon and its Compounds
A metal nitrate 'A' on heating gives a metal oxide along with evolution of a brown coloured gas 'B' and a colourless gas, which helps in burning. Aqueous solution of 'A' when reacted with potassium iodide forms a yellow precipitate.
- Identify 'A' and 'B'
- Name the types of the reactions involved in the above statement.
Chapter: [0.01] Chemical Reactions and Equations
List two differences between the movement of leaves of a sensitive plant and the movement of a shoot towards light.
Chapter: [0.06] Control and Co-ordination
What happens at the synapse between two neurons?
Chapter: [0.06] Control and Co-ordination
Give the name of the enzyme present in the fluid in our mouth cavity. State the gland which produces it. What would happen to the digestion process if this gland stops secreting this enzyme?
Chapter: [0.05] Life Processes
Advertisements
Three resistors of 6Ω, 4Ω and 4Ω are connected together so that the total resistance is 8Ω. Draw a diagram to show this arrangement and give reason to justify your answer.
Chapter: [0.11] Electricity
A light ray enters from medium A to medium B as shown in the figure.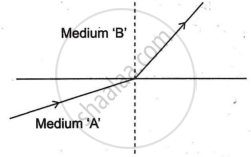
Which one of the two media is denser w.r.t. other medium? Justify your answer.
Chapter: [0.09] Light - Reflection and Refraction
A light ray enters from medium A to medium B as shown in the figure.
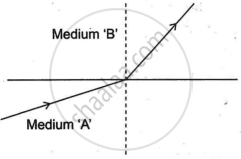
If the speed of light in medium A is `v_a` and in medium B is `v_b`, what is the refractive index of B with respect to A.
Chapter: [0.09] Light - Reflection and Refraction
A ray of light starting from diamond is incident on the interface separating diamond and water. Draw a labelled ray diagram to show. the refraction of light in this case.
Chapter: [0.09] Light - Reflection and Refraction
Absolute refractive indices of diamond and water are 2.42 and 1.33 respectively. Find the value of refractive index of water w.r.t. diamond.
Chapter: [0.09] Light - Reflection and Refraction
State the rule to determine the direction of a magnetic field produced around a straight conductor-carrying current.
Chapter: [0.12] Magnetic Effects of Electric Current
Name and state the rule of determine the direction of force experienced by a current carrying straight conductor placed in a uniform magnetic field which is perpendicular to it.
Chapter: [0.12] Magnetic Effects of Electric Current
Explain the process of transport of oxygenated and deoxygenated blood in a human body.
Chapter: [0.05] Life Processes
A substance 'X' is used as a building material and is insoluble in water. When it reacts with dil. HCl, it produces a gas which turns lime water milky.
- Write the chemical name and formula of 'X'.
- Write chemical equations for the chemical reactions involved in the above statements.
Chapter: [0.02] Acids, Bases and Salts
A metal 'M' on reacting with dilute acid liberates a gas 'G'. The same metal also liberates gas 'G' when reacts with a base.
- Write the name of gas 'G'.
- How will you test the presence of this gas?
- Write chemical equations for the reactions of the metal with (1) an acid and (2) a base.
Chapter: [0.02] Acids, Bases and Salts
Some plants like pea plants have tendrils which help them to climb up other plants. Explain how is it done. Name the plant hormone responsible for this movement.
Chapter: [0.06] Control and Co-ordination
Name the phenomenon occurring in plants which are under the control of gravity, water and chemicals with one example each that shows the movement involved.
Chapter: [0.06] Control and Co-ordination
With the help of an appropriate example, justify that some of the chemical reactions are determined by Change in temperature.
Give chemical equation for the reaction involved in the above case.
Chapter: [0.01] Chemical Reactions and Equations
With the help of an appropriate example, justify that some of the chemical reactions are determined by Evolution of a gas.
Give chemical equation for the reaction involved in the above case.
Chapter: [0.01] Chemical Reactions and Equations
With the help of an appropriate example, justify that some of the chemical reactions are determined by Change in colour
Give chemical equation for the reaction involved in the above case.
Chapter: [0.01] Chemical Reactions and Equations
A reddish brown metal used in electrical wires when powdered and heated strongly turns black. When hydrogen gas is passed over this black substance, it regains its original colour. Based on this information answer the following questions:
- Name the metal and the black substance formed.
- Write balanced chemical equations for the two reactions involved in the above information
Chapter: [0.01] Chemical Reactions and Equations
What is a solenoid?
Chapter: [0.12] Magnetic Effects of Electric Current
When does a solenoid behave as a magnet? Draw the pattern of the magnetic field produced inside it showing the directions of the magnetic field lines.
Chapter: [0.12] Magnetic Effects of Electric Current
If a harmful chemical enters a food chain comprising peacocks, plants, rats and snakes, which of these organisms is likely to have the highest concentration of the chemical in its body? Justify your answer. Name the process involved and define it.
Chapter: [0.13] Our Environment
Advertisements
- A compound 'A' with a molecular formula of \[\ce{C2H4O2}\] reacts with a base to give salt and water. Identify 'A', state its nature and the name of the functional group it possesses. Write chemical equation for the reaction involved.
- When the above stated compound 'A' reacts with another compound 'B' having molecular formula \[\ce{C2H6O}\] in the presence of an acid, a sweet smelling compound is 'C' formed.
- Identify 'B' and 'C'.
- State the role of acid in this reaction.
- Write chemical equation for the reaction involved.
Chapter: [0.02] Acids, Bases and Salts
Name the compound formed when ethanol is heated at 443 K in the presence of conc. \[\ce{H2SO4}\] and draw its electron dot structure.
State the role of conc. \[\ce{H2SO4}\] in the reaction.
Chapter: [0.04] Carbon and its Compounds
Explain hydrogenation with the help of a chemical equation. State the role of this reaction in industry.
Chapter:
Give reason for the following:
During reproduction inheritance of different proteins will lead to altered body designs.
Chapter: [0.07] How do Organisms Reproduce? [0.08] Heredity
Give reason for the following:
Fertilization cannot take place in flowers if pollination does not occur.
Chapter: [0.07] How do Organisms Reproduce?
Give reason for the following:
All multicellular organisms cannot give rise to new individuals through fragmentation or regeneration.
Chapter: [0.07] How do Organisms Reproduce?
Give reason for the following:
Vegetative propagation is practised for growing only some type of plants.
Chapter: [0.07] How do Organisms Reproduce?
Give reason for the following:
The parents and off-springs of organisms reproducing sexually have the same number of chromosomes.
Chapter: [0.07] How do Organisms Reproduce?
What is meant by resistance of a conductor ? Define its SI unit.
Chapter: [0.11] Electricity
On what factors does the resistance of a conductor depend?
Chapter: [0.11] Electricity
How will the resistance of a wire be affected if its
- length is doubled, and
- radius is also doubled ?
Give justification for your answer.
Chapter: [0.11] Electricity
Three incandescent bulbs of 100 W each are connected in series in an electric circuit. In another circuit another set of three bulbs of the same wattage are connected in parallel to the same source.
- Will the bulb in the two circuits glow with the same brightness? Justify your answer.
- Now let one bulb in both the circuits get fused. Will the rest of the bulbs continue to glow in each circuit? Give reason.
Chapter: [0.11] Electricity
What is meant by isomers?
Chapter: [0.04] Carbon and its Compounds
Write the structures of two compounds having molecular formula C3H6O and give their names.
Chapter: [0.04] Carbon and its Compounds
What is a soap?
Chapter: [0.04] Carbon and its Compounds
How is soap chemically different from detergents?
Chapter: [0.04] Carbon and its Compounds
Why do soaps not work effectively in hard water?
Chapter: [0.04] Carbon and its Compounds
What is meant by homologous series of carbon compounds?
Chapter: [0.04] Carbon and its Compounds
Write general formula for alkynes.
Chapter: [0.04] Carbon and its Compounds
Name and draw the electron dot structure of first homologue of alkynes series.
Chapter: [0.04] Carbon and its Compounds
State the meaning of the functional group in an organic compound.
Chapter:
Write the formula of the functional group present in alcohols.
Chapter:
Write the formula of the functional group present in carboxylic acids.
Chapter:
All human chromosomes ate not paired. Most human chromosomes have a maternal and a paternal copy, and we have 22 such pairs. But one pair called the sex chromosomes, is odd in not always being a perfect pair. Women have a perfect pair of sex chromosomes. But men have a mismatched pair in which one is normal sized while the other is a short one.
(a) In humans, how many chromosomes are present in a Zygote and in each gamete?
(b) A few reptiles rely entirely on environmental cues for sex determination. Comment.
(c) "The sex of a child is a matter of chance and none of the parents are considered to be responsible for it." Justify it through flow chart only.
OR
(c) Why do all the gametes formed in human females have an X chromosome?
Chapter: [0.08] Heredity
A student took three concave mirrors of different focal lengths and performed the experiment to see the image formation by placing an object at different distance with these mirrors as shown in the following table.
| Case No. | Object-distance | Focal length |
| I | 45 cm | 20 cm |
| II | 30 cm | 15 cm |
| III | 20 cm | 30 cm |
Now answer the following questions:
(a) List two properties of the image formed in Case I.
(b) In which one of the cases given in the table, the mirror will form real image of same size and why?
(c) Name the type of mirror used by dentists. Given reason why do they use such type of mirrors.
OR
(c) Look at the table and identify the situation (object distance and focal length) which resembles the situation in which concave mirrors are used as shaving mirrors? Draw a ray diagram to show the image formation in this case.
Chapter: [0.09] Light - Reflection and Refraction
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CBSE previous year question papers Class 10 Science with solutions 2022 - 2023
Previous year Question paper for CBSE Class 10 Science-2023 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Science, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CBSE Class 10.
How CBSE Class 10 Question Paper solutions Help Students ?
• Question paper solutions for Science will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
