Advertisements
Advertisements
प्रश्न
Writing a chemical reaction in brief by using chemical formulae is called as _______.
विकल्प
chemical change
chemical symbol
chemical equation
chemical reaction
उत्तर
Writing a chemical reaction in brief by using chemical formulae is called as chemical equation.
APPEARS IN
संबंधित प्रश्न
Write complete balanced equation for the following reaction:
Calcium (solid) + water (liquid)  Calcium Hydroxide (solution) + Hydrogen (gas)
Calcium Hydroxide (solution) + Hydrogen (gas)
What do you understand by endothermic reactions?
Is Respiration an endothermic reaction or an exothermic reaction?
Balance the following chemical equation:
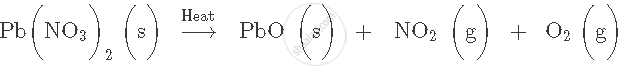
Write your observations and name the product when
Iron nails are added to an aqueous solution of copper sulphate.
Write word equation for the following skeletal equation:
\[\ce{CO + O2 -> CO2}\]
What information do you get from the equation H2+ Cl2 → 2HCl ?
Balance the following equation. Also name the product formed.
`"KCIO"_3 → "KCI" +"O"_2`
Balance the following equation. Also name the product formed.
` "H"_2"O"_2 → "H" _2 "O" + "O"_2`
Balance the following equation. Also name the product formed.
`"H"_2 +"CI"_2 → "HCI"`
Balance the equation stepwise.
H2S2O7(l) + H2O(l) → H2SO4(l)
Balance the equation stepwise.
SO2(g) + H2S(aq) → S(s) + H2O(l)
Balance the following equation:
Pb3O4 + HCl → PbCl2 + H2O + Cl2
Balance the following equation:
P + HNO3 → NO2 + H2O + H3PO4
What is the valency of :
carbon in CH4
Write the balanced chemical equation of the following reaction.
Potassium permanganate + hydrochloric acid → potassium chloride + manganese chloride + chlorine + water
Write the balanced chemical equation of the following reaction.
potassium dichromate + sulphuric acid → potassium sulphate + chromium sulphate + water + oxygen.
Write the balanced chemical equation of the following reaction.
potassium dichromate + hydrochloric acid → Potassium chloride + chromium chloride + water + chlorine
\[\ce{MnO2 + 4HCl -> MnCl2 + 2H2O + Cl2}\]
0.02 moles of pure MnO2 is heated strongly with conc. HCl. Calculate mass of MnO2 used.
A chemical reaction is generally accompanied by certain external indications or characteristics. These include – change of – (a) colour (b) state (c) smell (d) evolution of gas (e) formation of precipitate (f) evolution or absorption of heat. With reference to change of colour – state the change in colour seen when the following are heated – zinc carbonate.
In certain reaction a change of state is observed i.e. solid to liquid, liquid to gas etc. – State the change of state of the products – to give the respective reactant.
NH3 + HCl ⇌ NH4Cl
In certain reaction an insoluble solid called precipitate is formed. State the colour and name of the precipitate formed in the following reaction involving addition of:
Iron [III] chloride to ammonium hydroxide.
Representation of the results of a chemical change – is a chemical equation.
For the equation: FeCl3 + 3NH4OH 3NH4Cl + Fe(OH)3 ↓
Answer the following:
What is the indications of the arrow between the reactants and the products and of the arrow pointing downwards at the end.
Give reason for the following:
Silver salts are kept in dark coloured bottles.
What is meant by ‘reactants’ and ‘products’ in a chemical equation?
Give an example of a chemical equation in which two reactants form:
three product
\[\ce{2KClO3->[MnO2] 2KCl + 3O2[g] - is a balanced equation.}\]
Give a reason why the above equation is balanced.
Balance the following simple equation:
Mg + N2 → Mg3N2
Balance the following simple equation:
NO + O2 → NO2
Balance the following simple equation:
Fe + Cl2 → FeCl3
Balance the following simple equation:
NaHCO3 + H2SO4 → Na2SO4 + H2O + CO2
CaCO3 + 2HCl[dil.] → CaCl2 + H2O + CO2 [g]
State the information provided by the above chemical equation.
Write a balanced equation for the following word equation:
Potassium nitrate → Potassium nitrite + Oxygen
Underline the compound in the equation given below, it is incorrectly balanced and write the correct balancing for the same.
CaC2 + N2 → 2CaCN2 + C
Underline the compound in the equation given below, it is incorrectly balanced and write the correct balancing for the same.
NaOH + CO2 → Na2CO3 + H2O
Write scientific reason.
Adding zinc particles to a solution of copper sulphate makes the blue solution colorless.
Which of the following statements about the given reaction are correct?
`3"Fe"("s") + 4"H"_2"O"("g") -> "Fe"_3"O"_4("s") + 4"H"_2("g")`
- Iron metal is getting oxidised
- Water is getting reduced
- Water is acting as reducing agent
- Water is acting as oxidising agent
What is a reactant in a chemical equation?
