Advertisements
Advertisements
Define the following term:
Fuel cell
Concept: Electrochemical Cells
Which of the following cell was used in the Apollo space programme?
Concept: Fuel Cells
For a reaction A + B ⟶ P, the rate is given by
Rate = k [A] [B]2
How is the rate of reaction affected if the concentration of B is doubled?
Concept: Factors Influencing Rate of a Reaction
For a reaction A + B ⟶ P, the rate is given by
Rate = k [A] [B]2
What is the overall order of reaction if A is present in large excess?
Concept: Factors Influencing Rate of a Reaction
For a chemical reaction R → P, the variation in the concentration (R) vs. time (t) plot is given as:
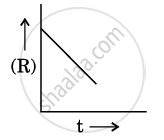
(i) Predict the order of the reaction.
(ii) What is the slope of the curve ?
(iii) Write the unit of rate constant for this reaction.
Concept: Factors Influencing Rate of a Reaction
Account for the following:
Mn shows the highest oxidation state of +7 with oxygen but with fluorine, it shows oxidation state of +4.
Concept: General Properties of the Transition Elements (D-block)
Account for the following:
Cu+2 salts are coloured, while Zn2+ salts are white.
Concept: General Properties of the Transition Elements (D-block)
Give a reason for the following:
Actinoids show irregularities in their electronic configurations.
Concept: F-block Elements > The Actinoids
Account for the following :
Zn is not considered as a transition element.
Concept: General Properties of the Transition Elements (D-block)
Give an example and suggest a reason for the following feature of the transition metal chemistry:
The lowest oxide of transition metal is basic, the highest is amphoteric/acidic.
Concept: Some Important Compounds of Transition Elements - Oxides and Oxoanions of Metals
Account for the following:
Mn2+ is more stable than Fe2+ towards oxidation to +3 state.
Concept: Electronic Configurations of the D-block Elements
Why \[\ce{HCl}\] should not be used for potassium permanganate titrations?
Concept: Some Important Compounds of Transition Elements - Oxides and Oxoanions of Metals
Write any two consequences of Lanthanoid Contraction.
Concept: F-block Elements > The Lanthanoids
Give reasons for the following statement:
\[\ce{Zn}\], \[\ce{Cd}\] and \[\ce{Hg}\] are soft metals.
Concept: General Properties of the Transition Elements (D-block)
Account for the following:
Transition metals form alloys.
Concept: General Properties of the Transition Elements (D-block)
Account for the following:
Sc3+ is colourless whereas Ti3+ is coloured in an aqueous solution.
Concept: General Properties of the Transition Elements (D-block)
What is the reason for the stability of colloidal sols?
Concept: Properties of Colloidal Solutions
Define the following term:
Zeta potential
Concept: Properties of Colloidal Solutions
[NiCl4]2− is paramagnetic, while [Ni(CO)4] is diamagnetic, though both are tetrahedral. Why? (Atomic number of Ni = 28)
Concept: Bonding in Coordination Compounds > Valence Bond Theory (VBT)
Write one similarity between Physisorption and Chemisorption
Concept: Types of Adsorption
