Topics
Circular Motion
- Angular Displacement
- Angular Velocity
- Angular Acceleration
- Angular Velocity and Its Relation with Linear Velocity
- Uniform Circular Motion (UCM)
- Radial Acceleration
- Dynamics of Uniform Circular Motion - Centripetal Force
- Centrifugal Forces
- Banking of Roads
- Vertical Circular Motion Due to Earth’s Gravitation
- Equation for Velocity and Energy at Different Positions of Vertical Circular Motion
- Kinematical Equations for Circular Motion in Analogy with Linear Motion.
Rotational Dynamics
- Rotational Dynamics
- Circular Motion and Its Characteristics
- Applications of Uniform Circular Motion
- Vertical Circular Motion
- Moment of Inertia as an Analogous Quantity for Mass
- Radius of Gyration
- Theorems of Perpendicular and Parallel Axes
- Angular Momentum or Moment of Linear Momentum
- Expression for Torque in Terms of Moment of Inertia
- Conservation of Angular Momentum
- Rolling Motion
Mechanical Properties of Fluids
- Fluid and Its Properties
- Thrust and Pressure
- Pressure of liquid
- Pressure Exerted by a Liquid Column
- Atmospheric Pressure
- Gauge Pressure and Absolute Pressure
- Hydrostatic Paradox
- Pascal’s Law
- Application of Pascal’s Law
- Measurement of Atmospheric Pressure
- Mercury Barometer (Simple Barometer)
- Open Tube Manometer
- Surface Tension
- Molecular Theory of Surface Tension
- Surface Tension and Surface Energy
- Angle of Contact
- Effect of Impurity and Temperature on Surface Tension
- Excess Pressure Across the Free Surface of a Liquid
- Explanation of Formation of Drops and Bubbles
- Capillarity and Capillary Action
- Fluids in Motion
- Critical Velocity and Reynolds Number
- Viscous Force or Viscosity
- Stokes’ Law
- Terminal Velocity
- Equation of Continuity
- Bernoulli's Equation
- Applications of Bernoulli’s Equation
Gravitation
- Newton’s Law of Gravitation
- Projection of Satellite
- Periodic Time
- Kepler’s Laws
- Binding Energy and Escape Velocity of a Satellite
- Weightlessness
- Variation of ‘G’ Due to Lattitude and Motion
- Acceleration Due to Gravity and Its Variation with Altitude and Depth
- Communication satellite and its uses
- Composition of Two S.H.M.’S Having Same Period and Along Same Line
Kinetic Theory of Gases and Radiation
- Gases and Its Characteristics
- Classification of Gases: Real Gases and Ideal Gases
- Mean Free Path
- Expression for Pressure Exerted by a Gas
- Root Mean Square (RMS) Speed
- Interpretation of Temperature in Kinetic Theory
- Law of Equipartition of Energy
- Specific Heat Capacity
- Absorption, Reflection, and Transmission of Heat Radiation
- Perfect Blackbody
- Emission of Heat Radiation
- Kirchhoff’s Law of Heat Radiation and Its Theoretical Proof
- Spectral Distribution of Blackbody Radiation
- Wien’s Displacement Law
- Stefan-boltzmann Law of Radiation
Angular Momentum
- Definition of M.I., K.E. of Rotating Body
- Rolling Motion
- Physical Significance of M.I (Moment of Inertia)
- Torque and Angular Momentum
- Theorems of Perpendicular and Parallel Axes
- M.I. of Some Regular Shaped Bodies About Specific Axes
Oscillations
- Periodic and Oscillatory Motion
- Simple Harmonic Motion (S.H.M.)
- Differential Equation of Linear S.H.M.
- Projection of U.C.M.(Uniform Circular Motion) on Any Diameter
- Phase of K.E (Kinetic Energy)
- K.E.(Kinetic Energy) and P.E.(Potential Energy) in S.H.M.
- Composition of Two S.H.M.’S Having Same Period and Along Same Line
- Some Systems Executing Simple Harmonic Motion
Thermodynamics
- Thermodynamics
- Thermal Equilibrium
- Zeroth Law of Thermodynamics
- Heat, Internal Energy and Work
- First Law of Thermodynamics
- Thermodynamic State Variables and Equation of State
- Thermodynamic Process
- Heat Engine
- Refrigerators and Heat Pumps
- Second Law of Thermodynamics
- Carnot Cycle and Carnot Engine
Elasticity
- Eneral Explanation of Elastic Property
- Plasticity
- Deformation
- Definition of Stress and Strain
- Hooke’s Law
- Elastic Energy
- Elastic Constants and Their Relation
- Determination of ‘Y’
- Behaviour of Metal Wire Under Increasing Load
- Application of Elastic Behaviour of Materials
Oscillations
- Oscillations
- Explanation of Periodic Motion
- Linear Simple Harmonic Motion (S.H.M.)
- Differential Equation of Linear S.H.M.
- Acceleration (a), Velocity (v) and Displacement (x) of S.H.M.
- Amplitude (A), Period (T) and Frequency (N) of S.H.M.
- Reference Circle Method
- Phase in S.H.M.
- Graphical Representation of S.H.M.
- Composition of Two S.H.M.’S Having Same Period and Along Same Line
- The Energy of a Particle Performing S.H.M.
- Simple Pendulum
- Angular S.H.M. and It's Differential Equation
- Damped Oscillations
- Free Oscillations, Forced Oscillations and Resonance Oscillations
- Periodic and Oscillatory Motion
Surface Tension
- Molecular Theory of Surface Tension
- Surface Tension
- Capillarity and Capillary Action
- Effect of Impurity and Temperature on Surface Tension
Superposition of Waves
- Superposition of Waves
- Progressive Waves
- Reflection of Waves
- Superposition of Waves
- Stationary Waves
- Free and Forced Vibrations
- Harmonics and Overtones
- Sonometer
- Beats
- Characteristics of Sound
- Musical Instruments
- The Speed of a Travelling Wave
- Speed of Wave Motion
- Study of Vibrations of Air Columns
Wave Motion
- Wave Motion Introduction
- Simple Harmonic Progressive Waves,
- Reflection of Transverse and Longitudinal Waves
- Change of Phase
- Principle of Superposition of Waves
- Formation of Beats
- Beats
Wave Optics
- Introduction of Wave Optics
- Nature of Light
- Light as a Wave
- Huygens’ Theory
- Reflection of Light at a Plane Surface
- Refraction of Light at a Plane Boundary Between Two Media
- Polarization
- Interference
- Diffraction of Light
- Resolving Power
Electrostatics
- Electrostatics
- Application of Gauss' Law
- Electric Potential and Potential Energy
- Electric Potential Due to a Point Charge, a Dipole and a System of Charges
- Equipotential Surfaces
- Electrical Energy of Two Point Charges and of a Dipole in an Electrostatic Field
- Conductors and Insulators, Free Charges and Bound Charges Inside a Conductor
- Dielectrics and Electric Polarisation
- Combination of Capacitors
- Displacement Current
- Energy Stored in a Capacitor
- Van De Graaff Generator
- Uniformly Charged Infinite Plane Sheet and Uniformly Charged Thin Spherical Shell (Field Inside and Outside)
Stationary Waves
- Study of Vibrations in a Finite Medium
- Formation of Stationary Waves on String
- Study of Vibrations of Air Columns
- Free and Forced Vibrations
- Forced Oscillations and Resonance
Kinetic Theory of Gases and Radiation
- Concept of an Ideal Gas
- Assumptions of Kinetic Theory of Gases
- Mean Free Path
- Derivation for Pressure of a Gas
- Degrees of Freedom
- Derivation of Boyle’s Law
- Thermal Equilibrium
- First Law of Thermodynamics
- Heat Engine
- Heat and Temperature
- Qualitative Ideas of Black Body Radiation
- Wien's Displacement Law
- Green House Effect
- Stefan's Law
- Maxwell Distribution
- Specific Heat Capacities - Gases
- Law of Equipartition of Energy
Current Electricity
- Current Electricity
- Kirchhoff’s Laws of Electrical Network
- Wheatstone Bridge
- Potentiometer
- Galvanometer
- Moving Coil Galvanometer
Magnetic Fields Due to Electric Current
- Magnetic Fields Due to Electric Current
- Magnetic Force
- Cyclotron Motion
- Helical Motion
- Magnetic Force on a Wire Carrying a Current
- Force on a Closed Circuit in a Magnetic Field
- Torque on a Current Loop in Magnetic Field
- Magnetic Dipole Moment
- Magnetic Potential Energy of a Dipole
- Magnetic Field Due to a Current: Biot-savart Law
- Force of Attraction Between Two Long Parallel Wires
- Magnetic Field Produced by a Current in a Circular Arc of a Wire
- Axial Magnetic Field Produced by Current in a Circular Loop
- Magnetic Lines for a Current Loop
- Ampere's Law
- Magnetic Field of a Solenoid and a Toroid
Wave Theory of Light
Interference and Diffraction
- Interference of Light
- Conditions for Producing Steady Interference Pattern
- Interference of Light Waves and Young’s Experiment
- Analytical Treatment of Interference Bands
- Measurement of Wavelength by Biprism Experiment
- Fraunhofer Diffraction Due to a Single Slit
- Rayleigh’s Criterion
- Resolving Power of a Microscope and Telescope
- Difference Between Interference and Diffraction
Magnetic Materials
- Magnetic Materials
- Torque Acting on a Magnetic Dipole in a Uniform Magnetic Field
- Origin of Magnetism in Materials
- Magnetisation and Magnetic Intensity
- Magnetic Properties of Materials
- Classification of Magnetic Materials
- Hysteresis
- Permanent Magnet and Electromagnet
- Magnetic Shielding
Electrostatics
- Applications of Gauss’s Law
- Mechanical Force on Unit Area of a Charged Conductor
- Energy Density of a Medium
- Dielectrics and Polarisation
- Concept of Condenser
- The Parallel Plate Capacitor
- Capacity of Parallel Plate Condenser
- Effect of Dielectric on Capacity
- Energy of Charged Condenser
- Condensers in Series and Parallel,
- Van-deGraaff Generator
Electromagnetic Induction
- Electromagnetic Induction
- Faraday's Laws of Electromagnetic Induction
- Lenz's Law
- Flux of the Field
- Motional Electromotive Force (e.m.f.)
- Induced Emf in a Stationary Coil in a Changing Magnetic Field
- Generators
- Back Emf and Back Torque
- Induction and Energy Transfer
- Eddy Currents
- Self Inductance
- Energy Stored in a Magnetic Field
- Energy Density of a Magnetic Field
- Mutual Inductance
- Transformers
Current Electricity
- Kirchhoff’s Rules
- Wheatstone Bridge
- Meter Bridge
- Metre Bridge
- Potentiometer
AC Circuits
- AC Circuits
- A.C. Generator
- Average and RMS Values
- Phasors
- Different Types of AC Circuits: AC Voltage Applied to a Resistor
- Different Types of AC Circuits: AC Voltage Applied to an Inductor
- Different Types of AC Circuits: AC Voltage Applied to a Capacitor
- Different Types of AC Circuits: AC Voltage Applied to a Series LCR Circuit
- Power in AC Circuit
- LC Oscillations
- Electric Resonance
- Sharpness of Resonance: Q Factor
- Choke Coil
Magnetic Effects of Electric Current
Dual Nature of Radiation and Matter
- Dual Nature of Radiation and Matter
- The Photoelectric Effect
- Wave-particle Duality of Electromagnetic Radiation
- Photo Cell
- De Broglie Hypothesis
- Davisson and Germer Experiment
- Wave-particle Duality of Matter
Magnetism
Structure of Atoms and Nuclei
- Structure of Atoms and Nuclei
- Thomson’s Atomic Model
- Geiger-marsden Experiment
- Lord Rutherford’s Atomic model
- Atomic Spectra
- Bohr’s Atomic Model
- Atomic Nucleus
- Constituents of a Nucleus
- Isotopes, Isobars and Isotones
- Atomic and Nuclear Masses
- Size and Density of the Nucleus
- Mass Defect and Binding Energy
- Binding Energy Curve
- Nuclear Energy
- Nuclear Binding Energy
- Radioactive Decays
- Law of Radioactive Decay
Semiconductor Devices
- Basics of Semiconductor Devices
- p-n Junction Diode as a Rectifier
- Special Purpose Junction Diodes
- Bipolar Junction Transistor (BJT)
- Basics of Logic Gates
Electromagnetic Inductions
- Electromagnetic Induction
- Faraday’s Law of Induction
- Self Inductance
- Mutual Inductance
- Transformers
- Need for Displacement Current
- Coil Rotating in Uniform Magnetic Induction
- Alternating Currents
- Reactance and Impedance
- LC Oscillations
- Inductance and Capacitance
- Resonant Circuits
- Power in AC Circuit: the Power Factor
- Lenz’s Law and Conservation of Energy
Electrons and Photons
Atoms, Molecules and Nuclei
- Alpha-particle Scattering and Rutherford’s Nuclear Model of Atom
- Bohr’s Model for Hydrogen Atom
- Hydrogen Spectrum
- Atomic Masses and Composition of Nucleus
- Introduction of Radioactivity
- Law of Radioactive Decay
- Atomic Mass, Mass - Energy Relation and Mass Defect
- Nuclear Binding Energy
- Nuclear Fusion – Energy Generation in Stars
- de-Broglie Relation
- Wave Nature of Matter
- Wavelength of an Electron
- Davisson and Germer Experiment
- Continuous and Characteristics X-rays
- Mass Defect and Binding Energy
Semiconductors
- Energy Bands in Solids
- Extrinsic Semiconductor
- Applications of n-type and p-type Semiconductors
- Special Purpose P-n Junction Diodes
- Semiconductor Diode
- Zener Diode as a Voltage Regulator
- I-V Characteristics of Led
- Transistor and Characteristics of a Transistor
- Transistor as an Amplifier (Ce-configuration)
- Transistor as a Switch
- Oscillators
- Digital Electronics and Logic Gates
Communication Systems
- Elements of a Communication System
- Basic Terminology Used in Electronic Communication Systems
- Bandwidth of Signals
- Bandwidth of Transmission Medium
- Need for Modulation and Demodulation
- Production and Detection of an Amplitude Modulated Wave
- Space Communication
- Propagation of Electromagnetic Waves
- Modulation and Its Necessity
- Surface Tension
- Force due to surface tension
- Factors affecting surface tension
1) Nature of liquid
2) Impurities
3) Temperature
4) Electrification - Applications of surface tension
Notes
Surface Energy
-
Surface energy is the excess energy exhibited by the liquid molecules on the surface compared to those inside the liquid.
-
This means liquid molecules at the surface have greater energy as compared to molecules inside it.
-
Suppose there is a tumbler and when we pour water in the tumbler, it takes the shape of the tumbler.
-
It acquires free surface.
Case 1: When molecules are inside the liquid:-
-
Suppose there is a molecule inside the water, there will be several other molecules that will attract that molecule in all the directions.
-
As a result this attraction will bind all the molecules together.
-
This results in negative potential energy of the molecule as it binds the molecule.
-
To separate this molecule huge amount of energy is required to overcome potential energy.
-
Some external energy is required to move this molecule and it should be greater than the potential energy.
-
Therefore in order to separate this molecule a huge amount of energy is required.
Case2: When the molecules are at the surface:-
- When the molecule is at the surface, half of it will be inside and half of it is exposed to the atmosphere.
-
For the lower half of the molecule it will be attracted by the other molecules inside the liquid.
-
But the upper half is free. The negative potential energy is only because of lower half.
-
But the magnitude is half as compared to the potential energy of the molecule which is fully inside the liquid.
-
So the molecule has some excess energy, because of this additional energy which the molecules have at the surface different phenomenon happen like surface energy, surface tension.
-
Liquids always tend to have least surface are when left to itself.
-
As more surface area will require more energy as a result liquids tend to have least surface area.
Surface energy for two fluids in contact:
-
Whenever there are two fluids, in contact, surface energy depends on materials of the surfaces in contact.
-
Surface energy decreases if the molecules of the two fluids attract.
-
Surface energy increases if molecules of the two fluids repel.
Surface Tension
-
Surface tension is the property of the liquid surface which arises due to the fact that surface molecules have extra energy.
-
Surface energy is the extra energy that the molecules at the surface have.
-
Surface tension is the property of the liquid surface because the molecules have extra energy.
-
Surface energy is defined as surface energy per unit area of the liquid surface.
-
Denoted by ’S’.
Mathematically:-
-
Consider a case in which liquid is enclosed in a movable bar.
-
Slide the bar slightly and it moves some distance (‘d’).
-
There will be increase in the area, (dl) where l=length of the bar.
-
Liquids have two surfaces one on the bar and others above the bar. Therefore area=2(dl)
-
Work done for this change =F x displacement.
-
Surface tension(S)=`"Surface Energy"/"area"`
-
Or Surface Energy=S x area
-
=S x 2dl
-
Therefore S x 2dl =F x d
-
`"S" = "F"/"2d"`
-
Surface tension is the surface energy per unit area of the liquid surface.
-
It can be also defined as Force per unit length on the liquid surface.
-
Important: -At any interface (it is a line that separates two different medium) the surface tension always acts in the equal and opposite direction and it is always perpendicular to the line at the interface.
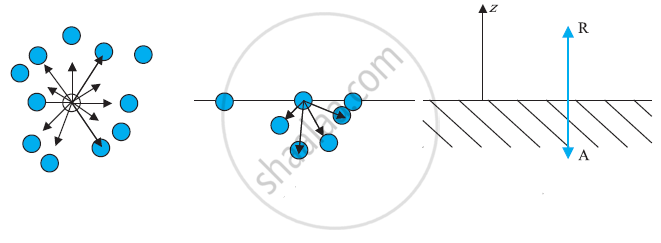 a) A molecule inside a liquid. Forces on a molecule due to others are shown. The direction of arrows indicates the attraction of repulsion. (b) Same, for a molecule at a surface. (c) Balance of attractive (A) and repulsive (R) forces.
a) A molecule inside a liquid. Forces on a molecule due to others are shown. The direction of arrows indicates the attraction of repulsion. (b) Same, for a molecule at a surface. (c) Balance of attractive (A) and repulsive (R) forces.
Surface tension and Surface energy: practical applications
-
Consider a molecule that is present completely inside the liquid and if it is strongly attracted by the neighboring molecules then the surface energy is less.
-
Consider a molecule that is present partially inside the liquid the force of attraction by the neighboring molecules is lesser as a result surface energy is more.
-
Consider a molecule whose very little part is inside the water so very small force of attraction by the neighboring molecules as a result of more surface energy.
-
Conclusion: - A fluid will stick to a solid surface if the surface energy between fluid and solid is smaller than the sum of energies between solid-air and fluid-air.
This means Ssf( solid-fluid) < Sfa(fluid air) + Ssa(Solid air)
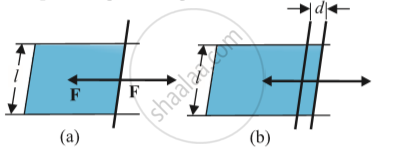
Stretching a film:
(a) A film in equilibrium
(b) The film stretched an extra distance
Why does water stick to glass but Mercury doesn’t?
In case of water and glass, water sticks to glass because the surface energy of water and glass is less than the surface energy between water and air and between glass and air. `"S.E"_(("w"-"g"))< "S.E"_(("w"-"a"))+ "S.E"_(("g"-"a"))`
In case of mercury, surface energy between mercury and glass `"S.E"_(("m"-"g"))`, surface energy between mercury and air `"S.E"_((m-a))` and surface energy between air and glass `"S.E"_(("a"-"g")). "E"_(("m"-"g"))>"S.E"_(("m"-"a"))+"S.E"_(("a"-"g"))`
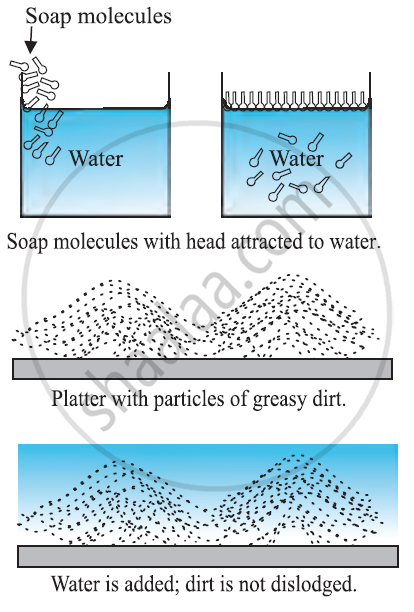
How detergents work?
-
Washing alone with the water can remove some of the dirt but it does not remove the grease stains. This is because water does not wet greasy dirt.
-
We need detergent which mixes water with dirt to remove it from the clothes.
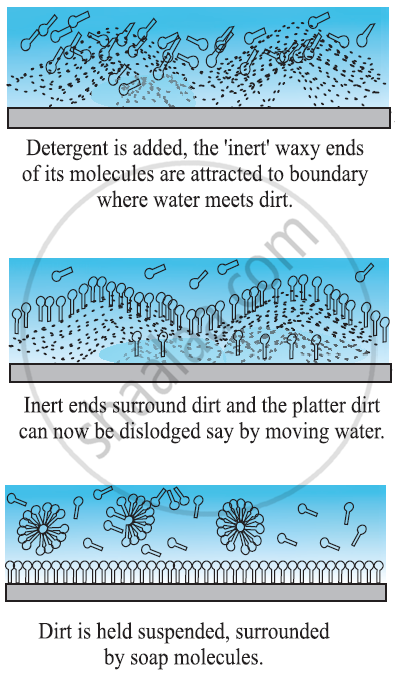
-
Detergent molecules look like hairpin shape. When we add detergents to the water one end stick to water and the other end sticks to the dirt.
-
As a result dirt is getting attracted to the detergent molecules and they get detached from the clothes and they are suspended in the water.
-
Detergent molecules get attracted to water and when water is removed the dirt also gets removed from the clothes.
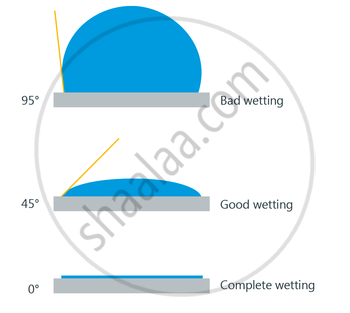
Angle of Contact:-
- The angle of contact is the angle at which a liquid interface meets a solid surface.
-
It is denoted by θ.
-
It is different at interfaces of different pairs of liquids and solids.
-
For example - Droplet of water on a lotus leaf. The droplet of water(Liquid) is in contact with the solid surface which is a leaf.
-
This liquid surface makes some angle with the solid surface. This angle is known as angle of contact.

Significance of Angle of Contact
-
Angle of contact determines whether a liquid will spread on the surface of a solid or it will form droplets on it.
-
If the Angle of contact is obtuse: then droplet will be formed.
-
If the Angle of contact is acute: then the water will spread.
-
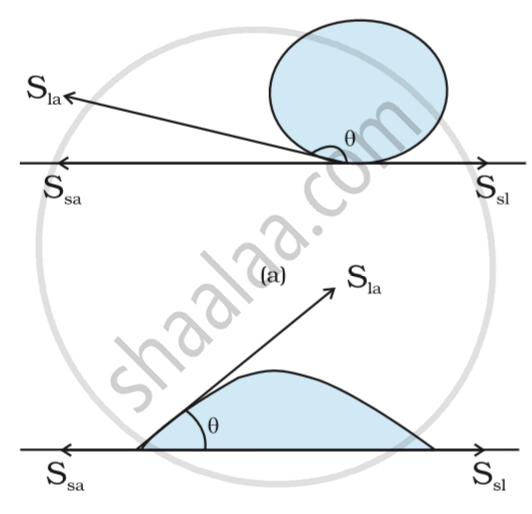
Case1: When droplet is formed
-
Consider we have a solid surface, droplet of water which is liquid and air.
-
The solid liquid interface denoted by Ssl, solid air interface denoted by Ssa and liquid air interface denoted by Sla.
-
The angle which Ssl makes with Sla. It is greater than the 900.
-
Therefore droplet is formed.
Case 2: When water just spreads
-
The angle which liquid forms with solid surface is less than 900.
Drops and Bubbles
Why water and bubbles are drops?
-
Whenever liquid is left to itself it tends to acquire the least possible surface area so that it has the least surface energy so it has the most stability.
-
Therefore for more stability, they acquire the shape of the sphere, as a sphere has the least possible area.
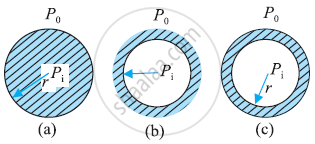
Distinction between Drop, Cavity, and Bubble:
Drop: - Drop is a spherical structure filled with water:
There is only one interface in the drop.
The interface separates water and air.
Example: Water droplet.
Water droplets:
Cavity:- Cavity is a spherical shape filled with air.
In the surroundings there is water and in the middle, there is a cavity filled with air.
There is only one interface which separates air and water.
Example:- bubble inside the aquarium.
Bubble:- In a bubble there are two interfaces. One is air-water and another is water and air.
Inside a bubble there is air and there is air outside.
But it consists of a thin film of water.
Pressure inside a drop and a cavity
-
The pressure inside a drop is greater than the pressure outside.
-
Suppose there is a spherical drop of water of radius ‘r’ which is in equilibrium.
-
Consider there is an increase in radius which is Δr.
Therefore extra surface energy = surface tension(S) x area
`=S_"l"a xx 4pi(r+Deltar)^2-S_"l"a xx 4pir^2`
after calculating
extra surface energy = `8piDeltarS_la`
At equilibrium, extra surface energy = energy gain due to the pressure difference
`8pir Deltar S_la = (P_i-P_o)4pir^2xxDeltar`
where `P_i` = pressure inside the drop and `P_o` = pressure outside the drop.
After calculation`P_i - P_o=(2S_(la))/r`
Pressure inside a Bubble
-
Pressure inside a bubble is greater than the pressure outside.
- As bubble has 2 interfaces, `"P"_i-"P"_o=(4"S"_"la")/("r")`
- This is probably why you have to blow hard, but not too hard, to form a soap bubble. A little extra air pressure is needed inside!
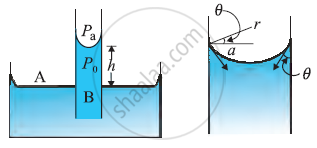
Capillary Rise
-
In Latin the word capilla means hair.
-
Due to the pressure difference across a curved liquid-air interface the water rises up in a narrow tube in spite of gravity.
-
Consider a vertical capillary tube of circular cross section (radius a) inserted into an open vessel of water.
-
The contact angle between water and glass is acute. Thus the surface of water in the capillary is concave. As a result there is a pressure difference between the two sides of the top surface. This is given by
`("P"_i-"P"_o)=("2S"/r)="2S"/(a sec theta)=("2S"/a) cos theta` ...(i)
Thus the pressure of the water inside the tube, just at the meniscus is less than the atmospheric pressure.
consider the two points A and B. They must be at the same pressure.
`"P"_0+"h"rho"g"="P"_i="P"_"A"` ...(ii)
Where `rho` is the density of water, and h is called the capillary.
By using eq (i) & (ii)
`"h"rho"g" = ("P"_i-"P"_o)=(2"S" cos theta)/a`
Therefore the capillary rise is due to surface tension. It is larger, for a smaller radius.
Problem:- The lower end of a capillary tube of diameter 2.00 mm is dipped 8.00 cm below the surface of the water in a beaker. What is the pressure required in the tube in order to blow a hemispherical bubble at its end in the water? The surface tension of water at temperature of the experiments is 7.30 ×10-2 Nm-1. 1 atmospheric pressure = 1.01 × 105 Pa, density of water = 1000 kg/m3, g = 9.80 m s-2.Also, calculate the excess pressure.
Answer:-
The excess pressure in a bubble of gas in a liquid is given by `"2S"/r`, where 'S' is the surface tension of the liquid-gas interface. As there is only one liquid surface, therefore using the formula pressure is `"4S"/r`. The radius of the bubble is 'r'. Now the pressure outside the bubble Po equals atmospheric pressure plus the pressure due to 8.00 cm of water column. That is
`"P"_o=(1.01xx105"Pa"+0.08"m"xx1000"kgm"^-3xx9.80"ms"^-2)`
`= 1.01784 xx 10^5 "Pa"`
Therefore, the pressure inside the bubble is
`"P"_i="P"_o + "2S"/"r"`
`1.01784 xx 10^5Pa +((2xx7.3xx10^-2)/10^-3 )`
`=(1.01784+0.00146)xx10^5"Pa"`
`=1.02xx10^5"Pa"` where the radius of the bubble is taken to be equal to the radius of the capillary tube since the bubble is hemispherical.
