Advertisements
Advertisements
प्रश्न
25 g of each of the following gases are taken at 27°C and 600 mm Hg pressure. Which of these will have the least volume?
पर्याय
HBr
HCl
HF
HI
उत्तर
HI
APPEARS IN
संबंधित प्रश्न
Which of the following is the correct expression for the equation of state of van der Waals gas?
Maximum deviation from ideal gas is expected from
Can a Van der Waals gas with a = 0 be liquefied? explain.
Suppose there is a tiny sticky area on the wall of a container of gas. Molecules hitting this area stick there permanently. Is the pressure greater or less than on the ordinary area of walls?
Explain whether a gas approaches ideal behavior or deviates from ideal behaviour if more gas is introduced into the same volume and at the same temperature.
Which of the following gases would you expect to deviate from ideal behavior under conditions of low-temperature F2, Cl2, or Br2? Explain.
Write the Van der Waals equation for a real gas. Explain the correction term for pressure and volume.
If 1 gram of each of the following gases are taken at STP, which of the gases will occupy (a) greatest volume and (b) smallest volume?
\[\ce{CO, H2O, CH4 , NO}\]
Isotherms of carbon dioxide gas are shown in figure. Mark a path for changing gas into liquid such that only one phase (i.e., either a gas or a liquid) exists at any time during the change. Explain how the temperature, volume and pressure should be changed to carry out the change.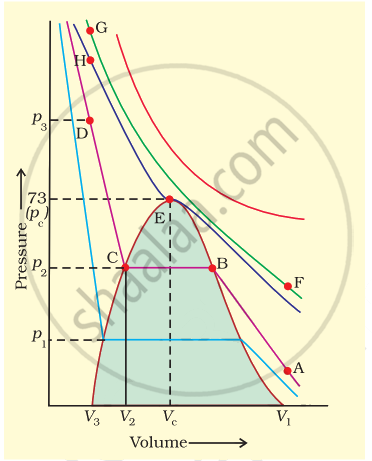
In van der Waal's equation for the real gas, the expression for the net force of attraction amongst the gas molecules is given by:
