Advertisements
Advertisements
प्रश्न
A geyser heats water flowing at the rate of 3.0 litres per minute from 27 °C to 77 °C. If the geyser operates on a gas burner, what is the rate of consumption of the fuel if its heat of combustion is 4.0 × 104 J/g?
उत्तर १
Water is flowing at a rate of 3.0 litre/min.
The geyser heats the water, raising the temperature from 27°C to 77°C.
Initial temperature, T1 = 27°C
Final temperature, T2 = 77°C
∴Rise in temperature, ΔT = T2 – T1
= 77 – 27= 50°C
Heat of combustion = 4 × 104 J/g
Specific heat of water, c = 4.2 J g–1 °C–1
Mass of flowing water, m = 3.0 litre/min = 3000 g/min
Total heat used, ΔQ = mc ΔT
= 3000 × 4.2 × 50
= 6.3 × 105 J/min
∴ Rate of consumption = `(6.3 xx 0^5)/(4xx10^4) = 15.75 "g/min"`
उत्तर २
Volume of water heated = 3.0 litre per minute Mass of water heated, m = 3000 g per minute Increase in temperature,
`triangle T = 77^@C - 27^@C =50^@C`
Specific heat of water, `c = 4.2 Jg^(-1) ""^@ C^(-1)`
Amount of heat used = `Q = mctriangleT`
or `Q = 3000 "gmin"^(-1) xx 4.2 Jg^(-1) ""^@C^(-1)xx50^@C`
`= 63 xx 10^4 J min^(-1)`
Rate of combustion of fuel = `(63xx10^4 J min^(-1))/(4.0xx10^4 Jg^(-1))` = 15.75 `"g min"^(-1)`
APPEARS IN
संबंधित प्रश्न
State two factors upon which the heat absorbed by a body depends
50 g of metal piece at 27°C requires 2400 J of heat energy so as to attain a temperature of 327°C . Calculate the specific heat capacity of the metal.
Heat energy is supplied at a constant rate to 100g of ice at 0 °C. The ice is converted into water at 0° C in 2 minutes. How much time will be required to raise the temperature of water from 0 °C to 20 °C? [Given: sp. heat capacity of water = 4.2 J g-1 °C-1, sp. latent heat of ice = 336 J g-1].
Give one example where high specific heat capacity of water is used as cooling purposes?
In an experiment to determine the specific heat capacity of a solid following operations were
made:
Mass of calorimeter + stirrer = x kg
Mass of water = y kg
Initial temperature of water t1℃
Mass of solid = z kg
Temperature of solid = t2 ℃
Temperature of mixture = t ℃
Specific heat capacity of calorimeter and water are c1 and c2 respectively. Express the specific
heat capacity c of the solid in terms of the above data.
How will global warming disturb the ecological balance?
How will you prove experimentally that different substances have different specific heat capacities?
Study the following procedure and answer the questions below:
1. Take 3 spheres of iron, copper and lead of equal mass.
2. Put all the 3 spheres in boiling water in a beaker for some time.
3. Take 3 spheres out of the water. Put them immediately on a thick slab of wax.
4. Note, the depth that each sphere goes into the wax.
i) Which property of substance can be studied with this procedure?
ii) Describe that property in minimum words.
iii) Explain the rule of heat exchange with this property.
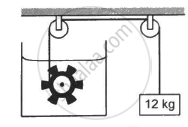
Figure shows a paddle wheel coupled to a mass of 12 kg through fixed frictionless pulleys. The paddle is immersed in a liquid of heat capacity 4200 J K−1 kept in an adiabatic container. Consider a time interval in which the 12 kg block falls slowly through 70 cm. (a) How much heat is given to the liquid? (b) How much work is done on the liquid? (c) Calculate the rise in the temperature of the liquid neglecting the heat capacity of the container and the paddle.
The substances like water which have ........... Heat capacity warm up more slowly than substances like iron which have .......... heat capacity.
A liquid X has specific heat capacity higher than the liquid Y. Which liquid is useful as coolant in car radiators.
What are other units of heat? Name and define them.
Why is specific heat capacity taken as a measure of thermal inertia?
Explain, why water is considered as best liquid for quenching thirst?
Solve the following problem.
Specific latent heat of vaporization of water is 2.26 × 106 J/kg. Calculate the energy needed to change 5.0 g of water into steam at 100 ºC.
All metals have the same specific heat capacity.
The specific heat capacity of water is ______.
Observe the following diagram and answer the questions given below:

Specific heat capacity of metals
- Which element has maximum specific heat capacity? Justify.
- Which element has minimum specific heat capacity? Justify.
- Define specific heat of object.
