Advertisements
Advertisements
प्रश्न
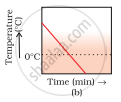
A glass tumbler containing hot water is kept in the freezer compartment of a refrigerator (temperature < 0°C). If you could measure the temperature of the content of the tumbler, which of the following graphs (Fig.1.2) would correctly represent the change in its temperature as a function of time.
पर्याय
उत्तर
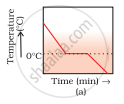
The hot water in the glass tumbler kept in the freezer will first become cold and the temperature will drop till 0°C. At 0°C, water loses heat equal to the latent heat of fusion till entire water freezes to form ice at 0°C. During this change of state from liquid to solid, the temperature remains constant.
On still further cooling, the temperature of ice slowly falls with time. Therefore, the correct option is (a).
APPEARS IN
संबंधित प्रश्न
Differentiate between melting point ,giving atleast one example of each.
Describe an experiment to demonstrate that a substance absorbs heat during melting without change in its temperature.
State the melting point of ice.
What are the changes of state in water? Explain.
Analogy:
Melting: ice into water on heating,
freezing: _______.
A student heats a beaker containing ice and water. He measures the temperature of the content of the beaker as a function of time. Which of the following (Fig. 1.1) would correctly represent the result? Justify your choice.
Water as ice has a cooling effect, whereas water as steam may cause severe burns. Explain these observations.
A change of state is a change of a substance from ______.
Explain the following effects of heat.
Change in state
Define the following:
Melting or fusion: